- Title
-
Comparative analysis of transcriptional changes in zebrafish cep290 and bbs2 mutants by RNA-seq reveals upregulation of inflammatory and stress-related pathways
- Authors
- Grabinski, S.E., Parsana, D., Perkins, B.D.
- Source
- Full text @ Front. Mol. Neurosci.
|
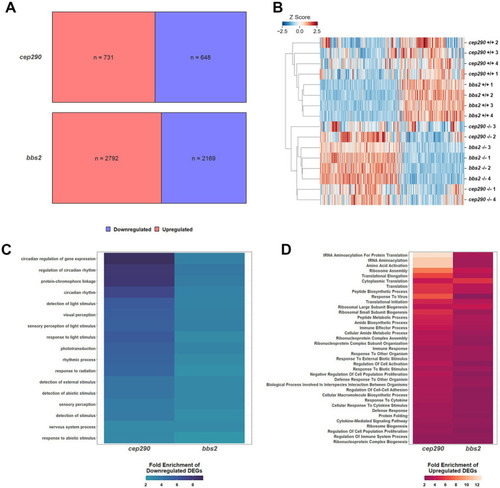
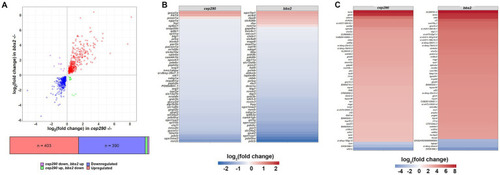
Differentially expressed genes (DEGs) in retinas of |
|
Enrichment of differentially expressed genes in biological processes of interest in |
|
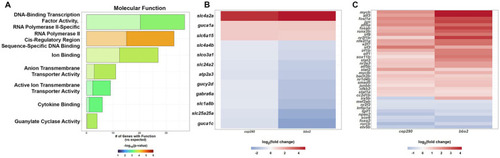
PANTHER Overrepresentation Analysis of the molecular function of the DEGs shared by |
|
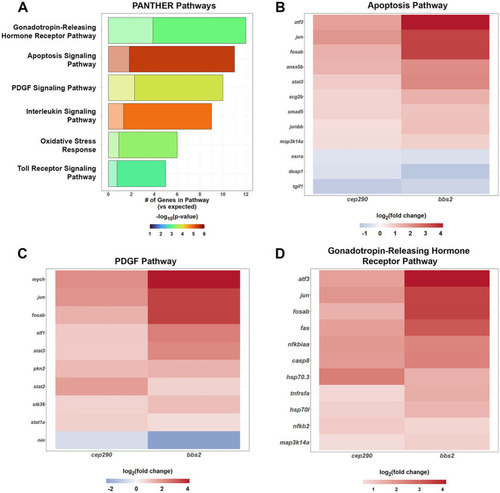
PANTHER Overrepresentation Analysis of the signaling pathways of the DEGs shared by |
|
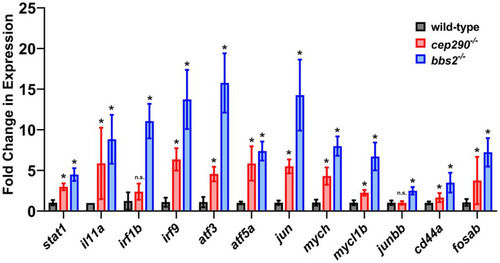
Fold change of gene expression in |
|
|