- Title
-
Control of Directed Cell Migration after Tubular Cell Injury by Nucleotide Signaling
- Authors
- Gessler, S., Guthmann, C., Schuler, V., Lilienkamp, M., Walz, G., Yakulov, T.A.
- Source
- Full text @ Int. J. Mol. Sci.
|
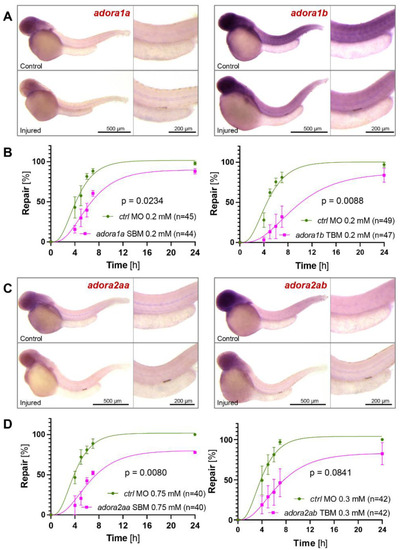
The involvement of adenosine receptors in zebrafish pronephros repair. ( |
|
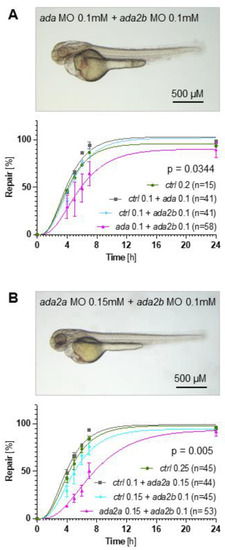
The involvement of adenosine deaminase family members in zebrafish pronephros repair. ( |
|
Synergistic effects between adenosine deaminase family members. ( PHENOTYPE:
|
|
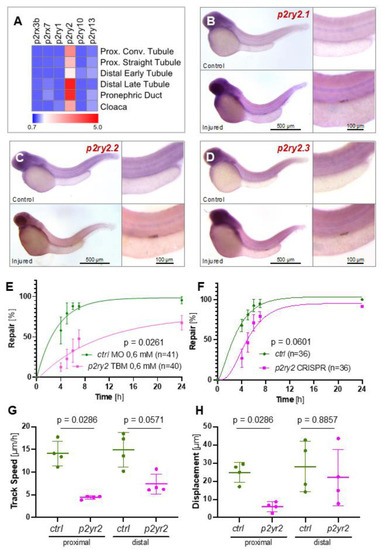
The involvement of purinergic P2ry2 receptors in zebrafish pronephros repair. ( |
|
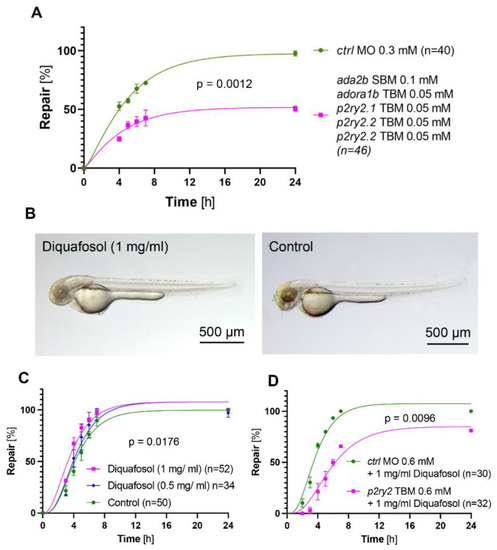
Effect of the combined adenosine pathway knockdown, and of the P2YR2 agonist Diquafosol on the repair process. ( PHENOTYPE:
|
|
Proposed ATP-dependent signaling after a laser-induced zebrafish pronephros injury. Damaged cells release nucleotides, including ATP. Released ATP is metabolized by ectonucleoside triphosphate diphosphohydrolase-1 (ENTPD1, CD39) and ecto-5′-nucleotidase (NT5E, CD73) to adenosine. Adenosine is rapidly removed from the extracellular environment by equilibrative nucleoside transporters (ENTs), or metabolized to inosine by extracellular adenosine deaminase (ADA), associated with CD26 or adenosine receptors. Intracellular adenosine is metabolized by cytoplasmic ADA. The G protein-coupled P2RY2 receptor, signaling through Gi/o, activates PLCß, while the adenosine A2A receptor stimulated adenylyl cyclase (AC) and cAMP production through Gs. Note that other adenosine family members couple to Gi/o, inhibiting AC. |