- Title
-
Human α-Defensin-6 Neutralizes Clostridioides difficile Toxins TcdA and TcdB by Direct Binding
- Authors
- Barthold, L., Heber, S., Schmidt, C.Q., Gradl, M., Weidinger, G., Barth, H., Fischer, S.
- Source
- Full text @ Int. J. Mol. Sci.
|
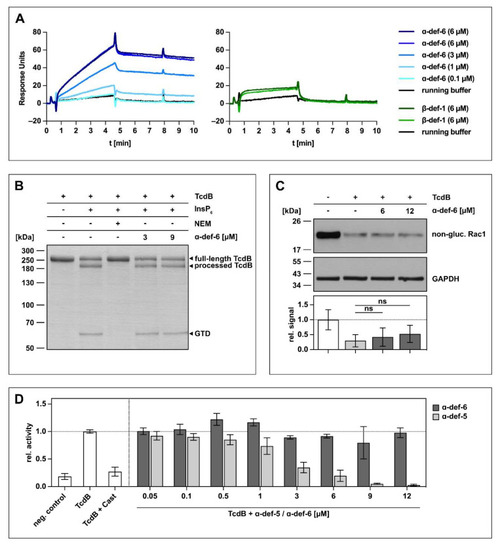
α-def-6 directly binds to TcdB in vitro but does not affect the autoproteolytic- and glucosyltransferase activity of TcdB. ( |
|
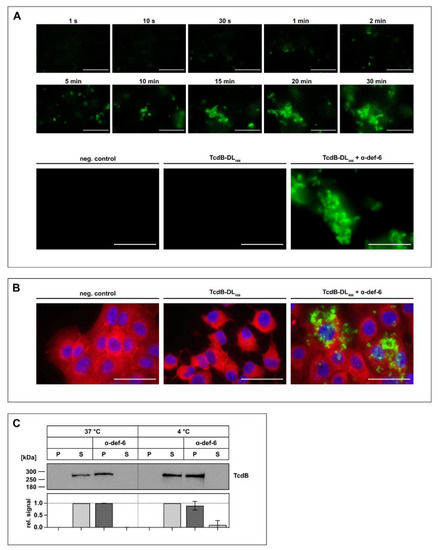
The incubation of α-def-6 and TcdB leads to rapid aggregation. ( |
|
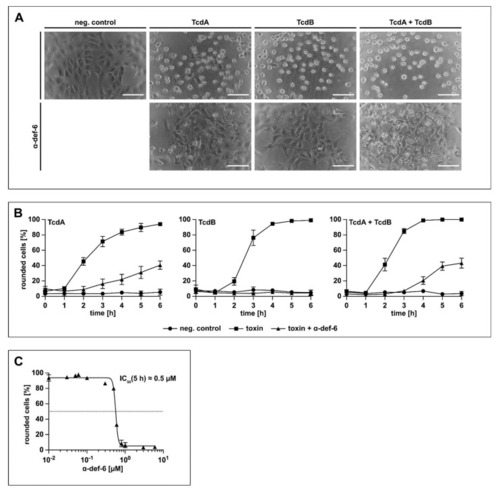
α-def-6 protects Vero cells from intoxication with TcdA, TcdB and the combination of both toxins. ( |
|
α-def-6 inhibits intracellular Rac1 glucosylation by TcdA, TcdB and the combination of both toxins. ( |
|
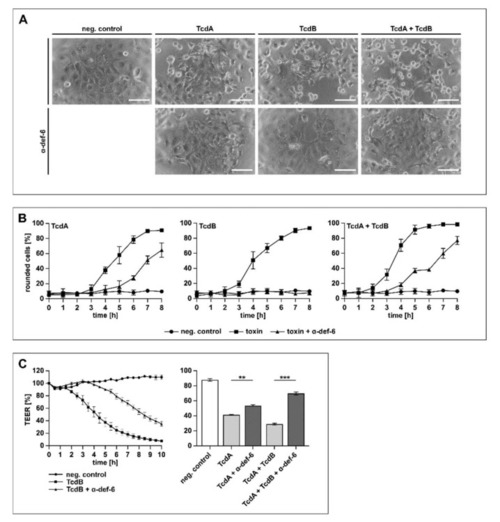
α-def-6 protects human CaCo-2 cells from intoxication with TcdA, TcdB and their combination. ( |
|
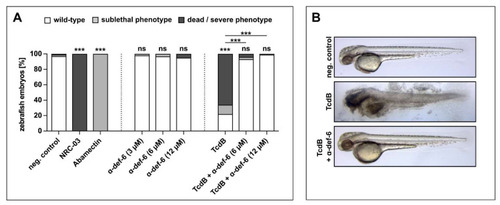
α-def-6 rescues zebrafish embryos from severe TcdB-induced damage and shows no self-toxicity. ( |