- Title
-
Müller Glia maintain their regenerative potential despite degeneration in the aged zebrafish retina
- Authors
- Martins, R.R., Zamzam, M., Tracey-White, D., Moosajee, M., Thummel, R., Henriques, C.M., MacDonald, R.B.
- Source
- Full text @ Aging Cell
|
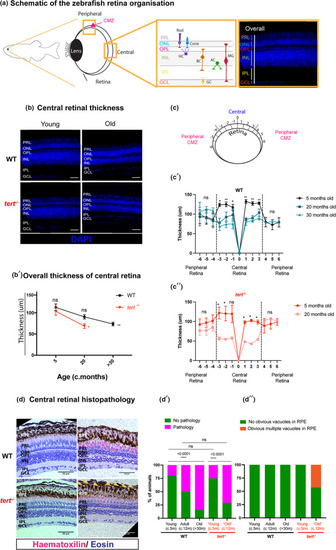
Zebrafish retina ageing is characterised by tissue thinning and morphological alterations, independently of telomerase. (a) Schematic figure of the zebrafish retina highlighting the peripheral, including the proliferative ciliary marginal zone (CMZ), and central retina, with respective layers and cell types (inset). (b) Central retina stained with DAPI in both WT and |
|
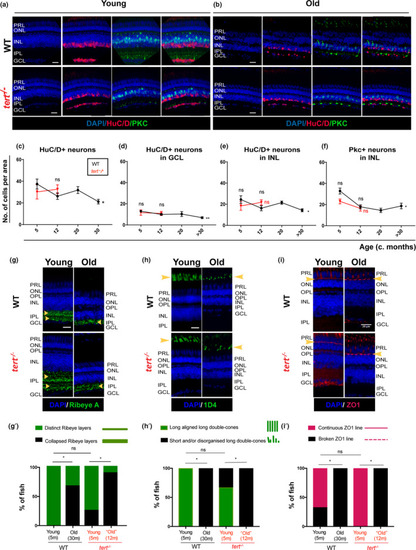
Zebrafish retina undergoes neurodegeneration with ageing, independently of telomerase. The central retina immunolabelled with HuC/D and PKC (amacrine in magenta and bipolar cells in green, respectively), in both WT and |
|
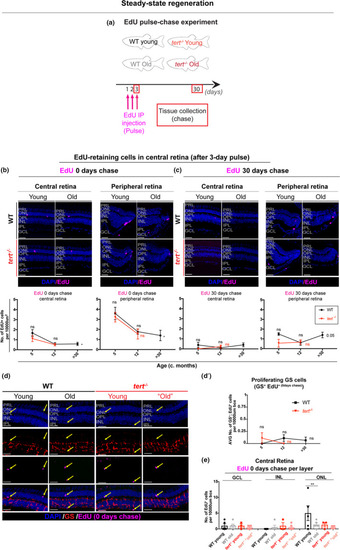
Aged zebrafish retina does not show signs of regeneration in response to spontaneous cell death and neuronal loss. (a) Schematic figure of the experimental design: 3‐day pulse of EdU, by IP injection, followed by 0‐ or 30‐day chase. We anticipate that in a healthy young fish the retina has some cells proliferating in the CMZ, which over‐time will replace older cells in the central retina. In the case of injury, we anticipate that there will be elevated levels of proliferation in peripheral and central retina to replace the dead cells. (b–c) The central and the peripheral retina immunolabelled with EdU (in purple), at (b) 0‐ and (c) 30‐day chase, in both WT and |
|
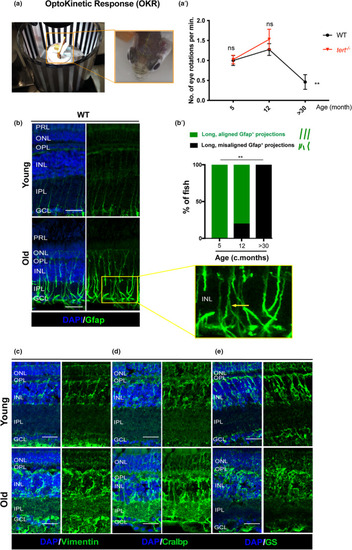
Zebrafish vision declines with ageing, which is accompanied by signs of glial morphological alterations. (a) OKR assay was performed by immobilising the fish in between soft sponges, inside a petri dish containing water, placed in the centre of a rotation chamber. The walls of the rotation chamber had 0.8 mm‐thick black and white stripes and the chamber was maintained at a constant velocity of 12 rpm throughout the experiment. (a’) The number of eye rotations per minute was manually quantified by video observation. Error bars represent the SEM. |
|
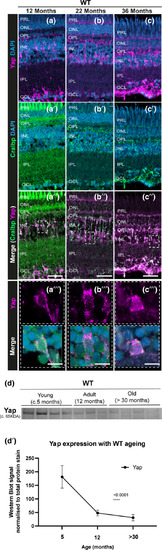
Yap expression is observed in the retinal MG throughout the zebrafish lifespan, but overall levels decrease with ageing. (a–c) The central retina immunolabelled for Yap expression (in magenta) and (a’–c’) Cralbp (MG cells, in green) in WT zebrafish at different ages (c. 12 months, 22 months, and 36 months). (a’’–c’’) Merge of DAPI, Yap and Cralbp staining and (a’’’–c’’’) respective inset showing Yap expression in MG cells at all stages observed. Representative images shown from |
|
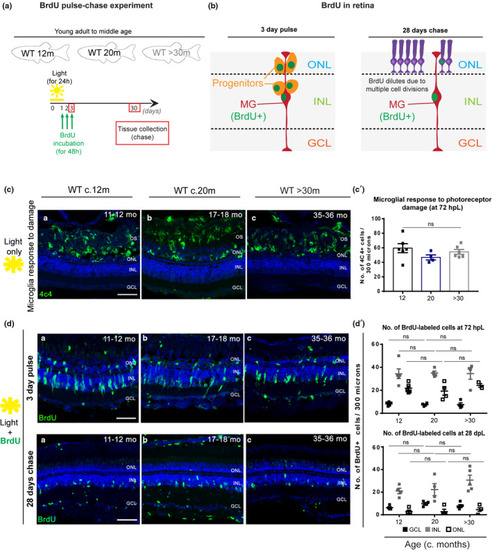
Zebrafish MG regenerative capacity upon acute damage is maintained in old age. (a) Schematic image of the experimental design and (b) expected results. Fish were light‐treated for 24 h and BrdU incorporation occurred between 24–72 h (green), which allowed for BrdU to be washed out the proliferating progenitors, leaving only MG which re‐entered the cell cycle to be labelled. (c) The central retina labelled with 4C4 (microglia, in green), after light treatment, in adults (c. 12 months), middle aged (c. 20 months) and old (>30 months) |