- Title
-
Zebrafish Vascular Mural Cell Biology: Recent Advances, Development, and Functions
- Authors
- Ando, K., Ishii, T., Fukuhara, S.
- Source
- Full text @ Life (Basel)
|
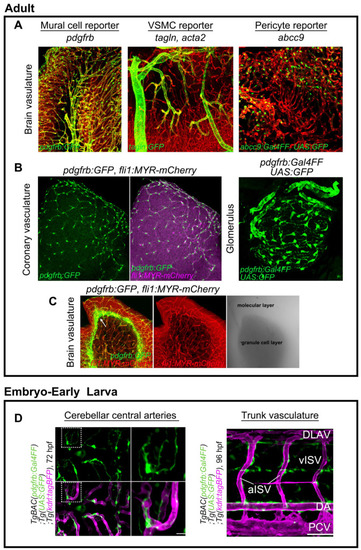
Transgenic zebrafish fluorescent reporter lines for mural cells. (A) Expressions of TgBAC(pdgfrb:GFP)ncv22Tg (left), TgBAC(tagln:EGFP)ncv25Tg (center), and TgBAC(abcc9:GAL4FF)ncv34Tg (right) reporters in zebrafish adult brain. Vessels are labeled with Tg(fli1:MYR-mCherry)ncv1Tg or |
|
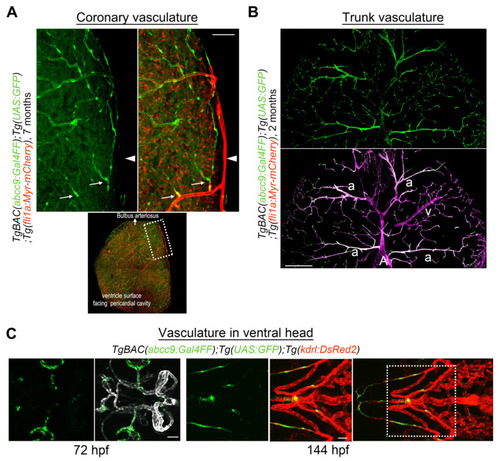
abcc9 reporter expression in the zebrafish. (A) Expression of TgBAC(abcc9:GAL4FF)ncv34Tg (green) is selective in capillary pericytes in coronary vessels. abcc9 reporter expression is negative around arteries (arrowhead) but starts after the appearance of several branches (arrows). Dotted area on the ventricle surface facing the pericardial cavity is enlarged at the top. (B) Transverse sectional view of the trunk vessels. In contrast to the brain (Figure 1A) and coronary vessels (Figure 2A), abcc9 is positive in arteriolar VSMC (a), while still being negative in arterial VSMC covering the primary artery (A) or the dorsal aorta (not shown in this image). Interestingly, the expression of abcc9 is lower in veins (v) than in arterioles or capillaries, which is also in clear contrast to the brain vasculature, suggesting that abcc9 expression reflects organotypic differences among mural cells. (C) Observation of the TgBAC(abcc9:GAL4FF)ncv34Tg reporter (green) around the vasculature in the ventral head at 72 hpf (left) or 144 hpf (right) indicates that mural cells covering the ventral artery and aortic arches are negative for abcc9, which is similar to the findings in the dorsal aorta. Dotted area in the right image is enlarged on the left. Scale bars: 50 μm (A), 200 μm (B), 30 μm (C, 72 hpf), 50 μm (C, 144 hpf). |
|
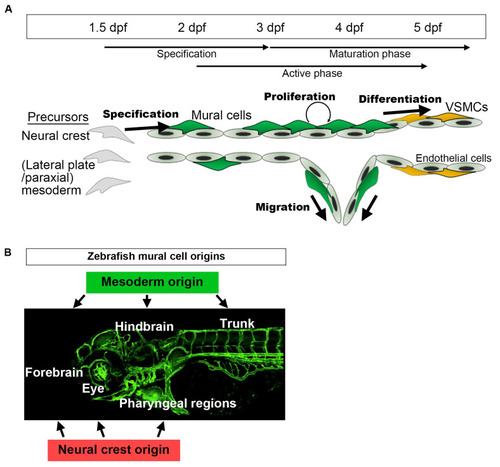
A schematic presentation of the developmental time course of zebrafish mural cells mainly in the brain and trunk. (A) Neural crest or mesoderm-derived mural cell precursors located in the vicinity of (arterial) endothelial cells are destined for the mural cell lineage during the period 36–72 hpf. Subsequently, such mural cells actively proliferate and migrate to cover arterial vessels. This proliferation and migration of mural cells appears to take place when active angiogenesis is induced (active phase). Along with the establishment of a vascular hierarchy, mural cells on larger caliber vessels start to differentiate into VSMCs after approximately 72 hpf (maturation phase). Upon formation of the vascular system, mural cell proliferation, migration, and differentiation take place. Mural cells, other than those on the dorsal/ventral aorta, resemble pericytes when they first appear, especially in the brain. However, whether they are identical to pericytes or still undergoing maturation (progenitor) to differentiate into VSMCs or pericytes remains unknown. (B) Mural cells in the hindbrain and trunk are of mesoderm origin, and those in pharyngeal regions and eyes are from the neural crest. The anterior part of the brain (forebrain) contains mural cells derived from both the mesoderm and the neural crest [12]. |
|
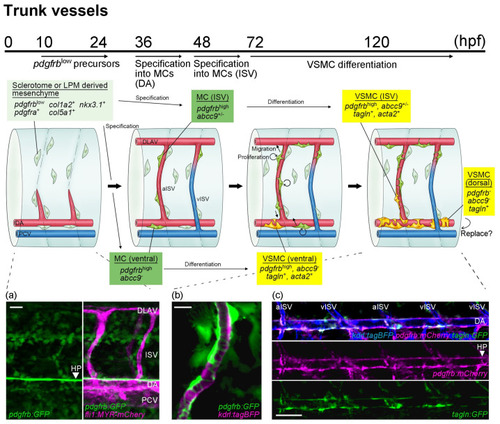
A schematic presentation of mural cell development in trunk vessels during early developmental stages. Potential pdgfrblow mural cell precursors (light green) are detectable from 10 hpf and are distributed throughout the trunk by 24 hpf, preferentially along the somite boundary where ISV is formed (a). Then, specification from pdgfrblowprecursors located in the vicinity of arterial endothelial cells into mural cells (green) starts after approximately 36 hpf beneath the dorsal aorta and 48 hpf on the ISVs. Subsequently, the proliferation and migration of mural cells are both induced in a PDGFRβ signaling-dependent manner. The arterial ISV (aISV) (b), the dorsal aorta (c), and the dorsal portion of the venous ISV (vISV) are well covered by mural cells by 96 hpf. Mural cells beneath the dorsal aorta start to wrap around the dorsal aorta and express VSMC markers such as acta2 and tagln at approximately 72 hpf. Later, these VSMC markers become positive in some ISV mural cells. In most mural cells differentiating into VSMC, acta2 expression is induced earlier than that of tagln. Images obtained in the vicinity of the dorsal aorta of TgBAC(pdgfrb:mCherry) ncv23Tg;TgBAC(tagln:GFP)ncv25Tg;Tg(kdrl:tagBFP)mu293Tg at 96 hpf are shown (b). Interestingly, the expression of tagln;GFP in mural cells increases when these cells are combined with pdgfrb reporter lines. Mural cells that are tagln-positive but negative for pdgfrb become apparent on the dorsal side of the dorsal aorta, mostly at approximately 96 hpf. However, whether VSMCs on the dorsal aorta consist of both VSMC sources or are replaced by mural cells emerging from the dorsal side, as observed in the mouse, remains to be determined. Scale bars: 20 μm (a,b), 50 μm (c). |
|
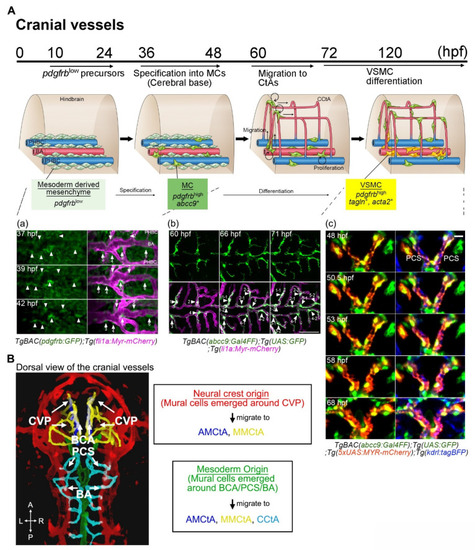
(A) A schematic of mural cell development in cranial vessels during early development from 10 hpf and are distributed at the cerebral base and around the CVP by 24 hpf. Then, specification from pdgfrblow precursors located in the vicinity of arterial endothelial cells on the BA, PCS, BCA, or CVP into mural cells (green) starts at approximately 36 hpf (a). Specification into mural cells from precursors is also slightly induced around primordial hindbrain channels (PHBCs). After the connection of CtAs to CVP, BCA, PCS, or BA and the start of blood circulation, mural cells emerging at the cerebral base migrate to CtAs. When these cells migrate, cell division frequently take place to entirely cover CtAs (b). Time-lapse imaging of TgBAC(abcc9:Gal4FF)ncv34Tg;Tg(UAS:GFP);Tg(5xUAS:MYR-mCherry)ncv504Tg;Tg(kdrl:tagBFP)mu293Tg shows morphological changes from pericyte-like cells to VSMCs around the PCS (c). Mural cell bodies and plasma membranes are highlighted by GFP (green) and MYR-mCherry (magenta), respectively. This VSMC-like morphological change in the cerebral base precedes the expression of VSMC markers such as acta2 or tagln. This is not always the case in CtA mural cells, however. (B) Mural cells appearing around the CVP are derived from the neural crest, whereas those emerging around the BCA, PCS, and BA have a mesodermal origin. Reflecting the connection routes of vessels and the features of mural cell migration along the vessels, mural cells around the CVP migrate toward AMCtAs (blue) and MMCtAs (yellow); those appearing at the BCA to AMCtAs and MMCtAs; and those emerging around the PCS or BA toward CCtAs (aqua). Therefore, mural cells in the zebrafish forebrain, in the hindbrain, or in the middle tend to become neural crest, mesoderm, or mixed-origin tissues, respectively. Scale bar: 20 μm (c). Choroidal vascular plexus (CVP). Basal communicating artery (BCA). Posterior communicating segment (PCS). Basilar artery (BA). Primordial hindbrain channel (PHBC). |
|
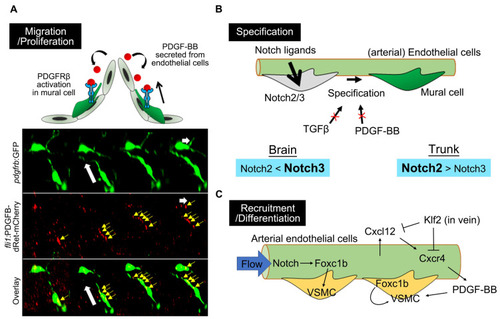
Molecular mechanisms underlying zebrafish mural cell development. (A) PDGF-BB/PDGFRβ signaling is essential for zebrafish mural cell migration and proliferation. The timelapse imaging of brain mural cells during the embryonic stage, at the bottom, shows the uptake of mCherry-fused PDGF-B into the mural cell processes (yellow arrows) extending toward the direction of migration. White arrows indicate the direction of mural cell migration. mCherry-fused PDGF-B depleted of the retention motif was originally expressed by endothelial cells using the fli1 promoter. This observation fits the proposed model in which PDGF-BB secreted from endothelial cells activates PDGFRβ expressed in mural cells to attract these cells to the vascular wall and the leading front. (B) Notch2/3 are both indispensable for mural cell specification, with a preference for Notch3 in the brain and Notch2 in the trunk. TGFβ or PDGF-BB is not essential for specification into mural cells, at least during early developmental stages, in the brain or trunk vessels of zebrafish. (C) Arterial endothelial cells induce VSMC recruitment or VSMC differentiation via Foxc1b or in a PDGF-BB-dependent manner. Cell-autonomous Foxc1b function, in VSMC developing from progenitors, has also been reported. |
|
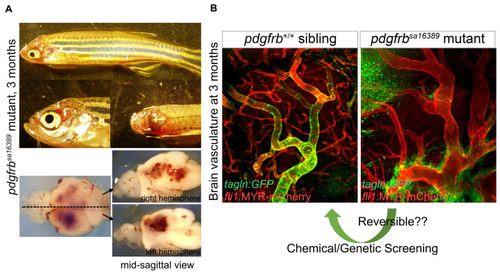
Abnormal vascular formation in pdgfrb mutant. (A) pdgfrbsa16389 mutants have no apparent vascular abnormalities, edema, or signs of hemorrhage during early developmental stages [53]. They start to show clear vascular abnormalities, however, after 1 month of age. After the juvenile stage, they have signs of bleeding (arrows) and the associated prominent phenotype in brain vessels. Images of brains dissected from the fish shown on the top are presented in the lower panels. (B) Comparison of the brain vasculature of wild-type (left) and pdgfrbsa16389 mutant (right) mice, showing the same anatomical region, confirms the capillary network reduction and dilation of arteries. Coverage with VSMC visualized by TgBAC(tagln:EGFP)ncv25Tg reporter (green) found in the wild type is absent in the pdgfrb mutant. Regardless of how severe vascular defects are, pdgfrb mutant zebrafish can reach adulthood and produce viable offspring, which is a unique feature of zebrafish and indicates the utility of the pdgfrb mutant zebrafish for chemical or genetic screening, allowing the discovery of treatments for vascular anomalies such as aneurysms. Scale bars: 50 μm (B). Fluorescent images are from [53]. |
|
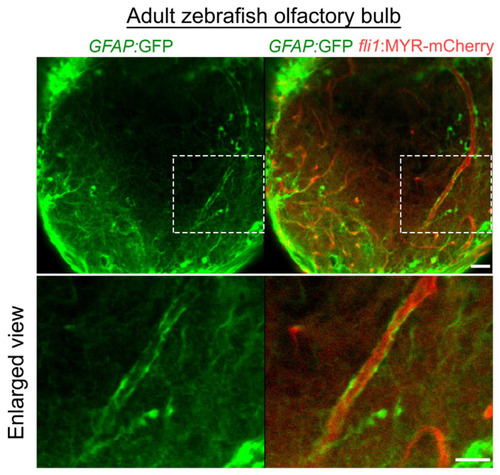
Distribution of astroglia in the zebrafish olfactory bulb. GFAP:GFP reporter (green) indicates vascular coverage by astroglia in the adult zebrafish olfactory bulb. Dotted areas are enlarged at the bottom. Scale bars: 30 μm, 20 μm (enlarged images). |