- Title
-
Ethylenediamine derivatives efficiently react with oxidized RNA 3' ends providing access to mono and dually labelled RNA probes for enzymatic assays and in vivo translation
- Authors
- Mamot, A., Sikorski, P.J., Siekierska, A., de Witte, P., Kowalska, J., Jemielity, J.
- Source
- Full text @ Nucleic Acids Res.
|
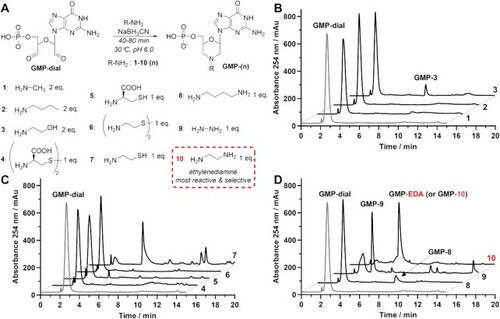
Reductive amination of GMP-dial with different N-nucleophiles (1–10). (A) Reaction scheme with general structure of reductive amination products GMP-(n). (B–D) HPLC chromatograms of crude reaction products after 40 min (1–6, 8) or 60 min (7, 9, 10) after addition of the nucleophile. During reaction between GMP-dial and butylamine (2) or cysteamine (7) indicated morpholine products (GMP-2 and GMP-7) were not detected. Product of reductive amination between GMP-dial and ethylenediamine (10, EDA) is designated as GMP-10 or GMP-EDA. |
|
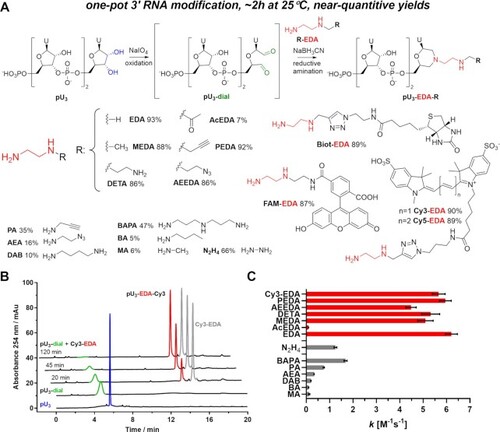
One-pot 3’-end RNA labelling. (A) Reaction scheme. Conditions: i) pU3 (140 µM), aqueous NaIO4 (1.4 mM), 30 min, and 25°C in the dark. ii) Addition of R-EDA (1.0 mM) and NaBH3CN (20 mM) in KH2PO4 buffer (0.1 M pH 6.0). HPLC-determined yields after two hours of reductive amination are presented for each N-nucleophile. (B) HPLC chromatograms of reaction with Cy3-EDA at different stages. (C) Second-order reaction rate constant k values determined for reductive amination reaction between pU3-dial (100 µM) and different N-nucleophiles (1 mM). |
|
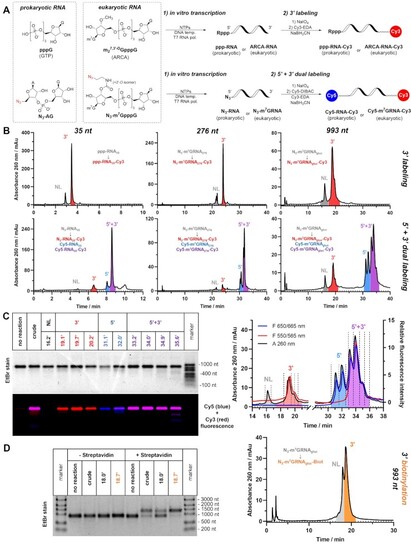
Mono- and dual labelling of IVT RNA. (A) Scheme of the process: NTPs mix in the presence or absence of initiating dinucleotide (ARCA, N3-AG or N3-m7GpppG) are incubated with gene-coding DNA template in presence of T7 RNA polymerase. After isolation from IVT mixture, RNA products are subjected to 3′ labelling (top) or simultaneous 5′+3′ dual labelling (bottom). (B) Representative HPLC chromatograms of crude labelling products of Cy3 3′ labelling (top) or Cy5/Cy3 5′+3′ dual labelling (bottom) performed on 35, 276 and 993 nucleotide-long RNAs (ppp-RNA35, N3-RNA35, N3-m7GRNA276 and N3-m7GRNAgluc). (C) Absorbance and fluorescence HPLC profiles of crude dual labelling product Cy5-m7GRNAgluc-Cy3 with designated retention times of collected fractions (right) and agarose electrophoresis of concentrated HPLC fractions (left); (D) HPLC chromatogram of crude 3′-biotinylated mRNA N3-m7GRNAgluc-Biot (right) and electrophoretic mobility shift assay (EMSA) of crude and HPLC-isolated products of 3′ biotinylation (left). |
|
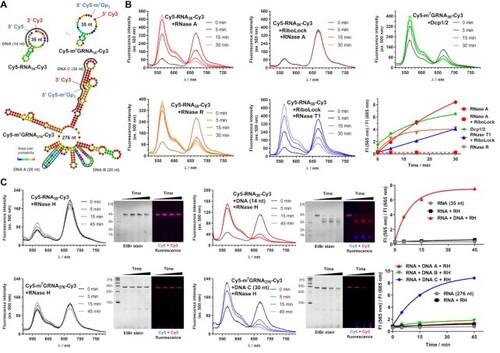
Application of dually labelled RNA probes for enzymatic activity monitoring. (A) Sequence and theoretical secondary structures of Cy5-RNA35-Cy3, Cy5-m7GRNA35-Cy3 and Cy5-m7GRNA276-Cy3 probes and illustration of studied DNA sequences complementary to regions of the probes. (B) Time-dependent changes of emission spectra and Cy3/Cy5 fluorescence intensity ratio after addition of RNase A, RiboLock and RNase A, RNase T1, RNase R or Dcp1/2 to Cy5-RNA35-Cy3 or Cy5-m7GRNA35-Cy3 probes. (C) Time-dependent changes of emission spectra, PAGE of reaction products, and Cy3/Cy5 fluorescence intensity ratio after addition of RNase H to probe (Cy5-RNA35-Cy3 or Cy5-m7GRNA276-Cy3) or DNA-probe duplex. |
|
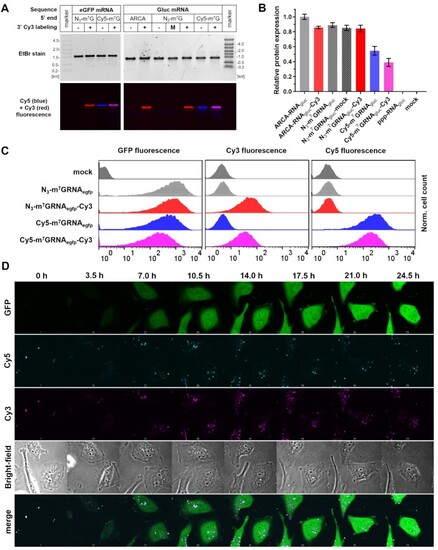
Fluorescent mRNA transfection into HeLa cells. (A) Electrophoretic resolution of HPLC-purified mRNAs encoding eGFP (N3-m7GRNAegfp, N3-m7GRNAegfp-Cy3, Cy5-m7GRNAegfp and Cy5-m7GRNAegfp-Cy3) or Gaussia luciferase (ARCA-RNAgluc, ARCA-RNAgluc-Cy3, N3-m7GRNAgluc, N3-m7GRNAgluc-mock, N3-m7GRNAgluc-Cy3, Cy5-m7GRNAgluc and Cy5-m7GRNAgluc-Cy3) used for HeLa transfections. (B) Relative expression of Gaussia luciferase after mRNA transfection as a function of the presence of 3′ Cy3 modification and 5′ cap structure. (C) Flow cytometry readouts after transfection of fluorescent mRNA encoding eGFP. (D) Time-lapse microscopy images of HeLa cells transfected with Cy5-m7GRNAegfp-Cy3 mRNA. Time scale set for 0 h at the beginning of recording of the images (1 h after transfection start). |
|
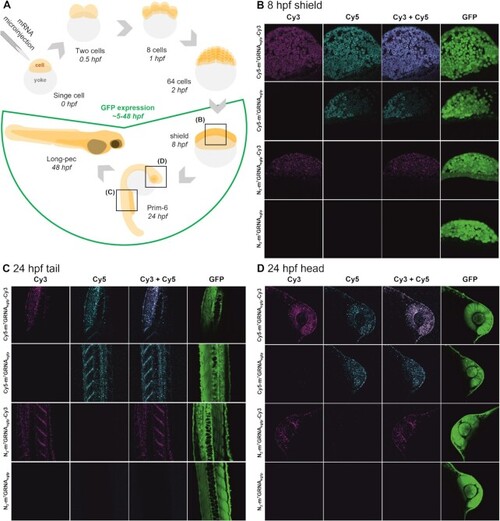
Microinjection of zebrafish embryos with modified mRNA. (A) Schematic representation of the experimental set-up and injection of mRNA in the course of zebrafish development during the first 48 h post fertilization (hpf). (B–D) Confocal microscopy images of embryos injected with 300 pg of N3-m7GRNAegfp, N3-m7GRNAegfp-Cy3, Cy5-m7GRNAegfp or Cy5-m7GRNAegfp-Cy3 mRNA captured at 8 hpf (B) or 24 hpf in tail (C) or head (D) sections. Bright field and GFP fluorescence images of whole embryos are presented in Supplementary Figure S24. |