- Title
-
Vestibular and Auditory Hair Cell Regeneration Following Targeted Ablation of Hair Cells With Diphtheria Toxin in Zebrafish
- Authors
- Jimenez, E., Slevin, C.C., Colón-Cruz, L., Burgess, S.M.
- Source
- Full text @ Front. Cell. Neurosci.
|
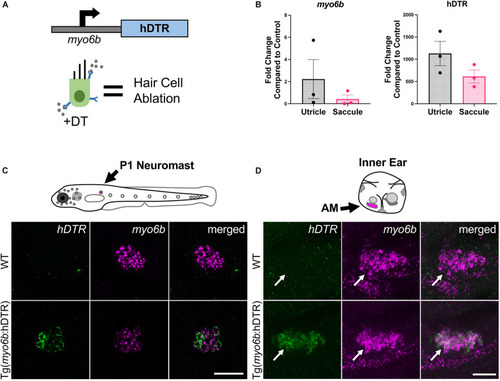
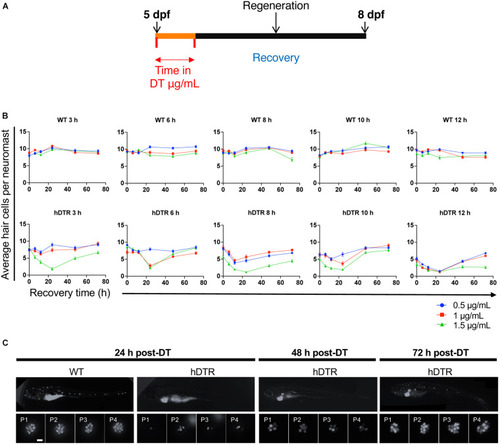
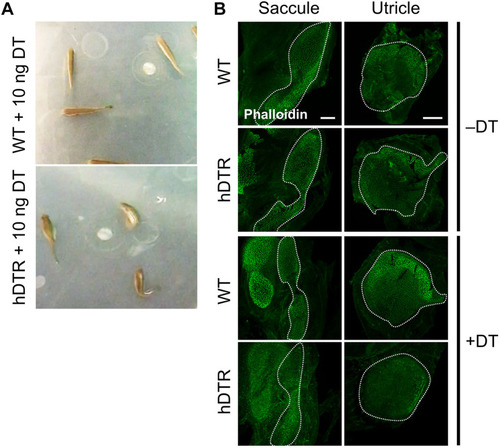
The Tg( |
|
Tg( |
|
Adult Tg( |
|
Tg( |
|
Tg( |
|
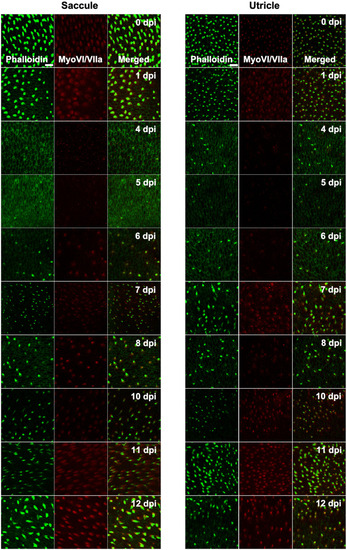
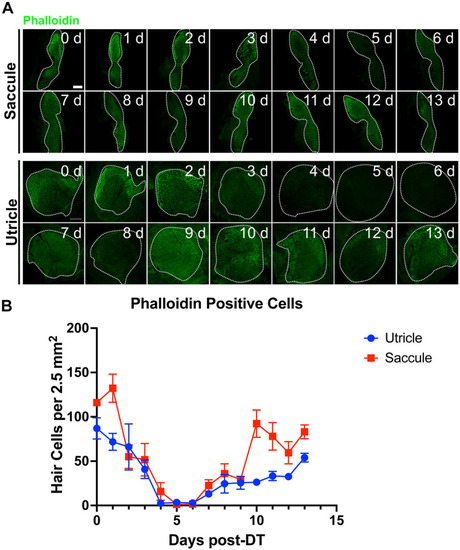
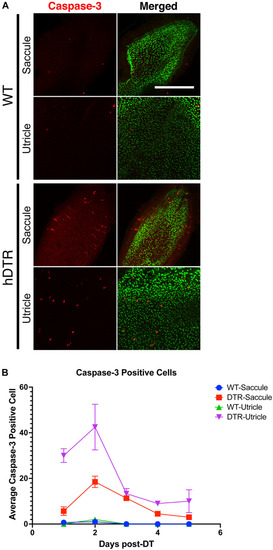
Figure 6. Diphtheria toxin induced hair cell death is primarily due to apoptosis. (A) Saccule and utricle from WT and Tg(myo6b:hDTR) (hDTR) inner ear tissues 2 days post-DT. Hair cells are labeled with phalloidin (green channel) and cleaved caspase-3 (red channel) positive cells were detected 2 days post-DT. (B) Quantification of cleaved caspase-3 positive cells in saccule and utricle dissected on days 1–5 following DT injection. Scale bar 100 μm. For qualitative purposes, brightness and contrast increased by 20%. |