- Title
-
A distributed saccade-associated network encodes high velocity conjugate and monocular eye movements in the zebrafish hindbrain
- Authors
- Leyden, C., Brysch, C., Arrenberg, A.B.
- Source
- Full text @ Sci. Rep.
|
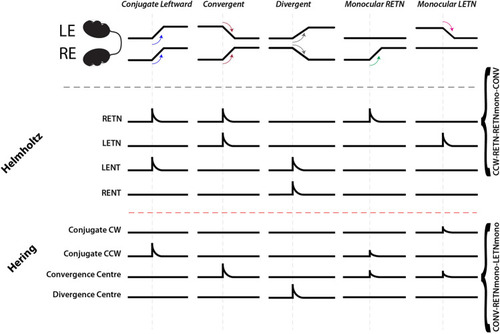
Schematic illustrating the burst neuron populations expected for neurons conforming to either the Hering or Helmholtz hypothesis, and their activity patterns. The descriptors along the right margin indicate how RETN neurons (Helmholtz) and Convergence Centre neurons (Hering) would be classified following our analysis (cf. Table S1). |
|
Measurement of cellular calcium signals during quick eye movements. ( |
|
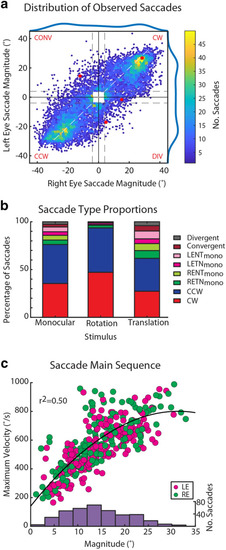
Characterization of binocular saccades. ( |
|
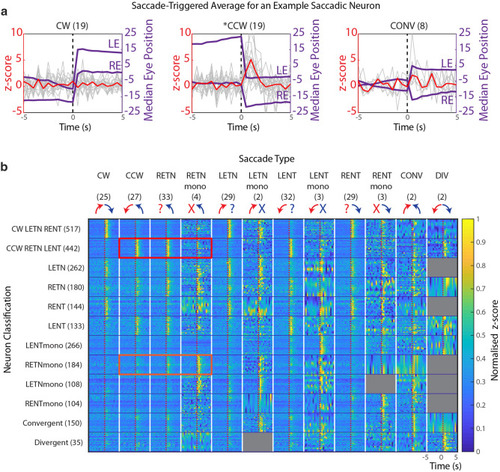
Peri-saccadic activity of different saccade-associated neuron types. ( |
|
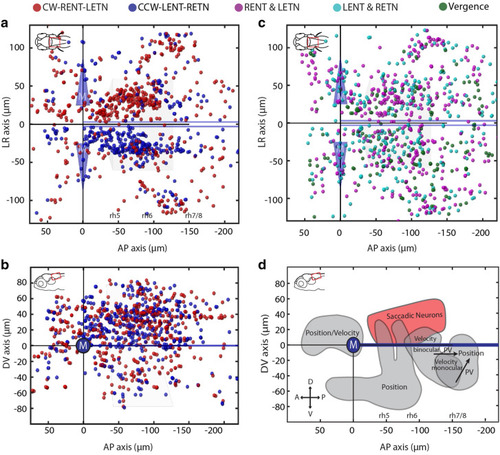
Anatomical distribution of putative burst neurons in the zebrafish hindbrain. Each colored ball represents an identified saccade-associated neuron. ( |
|
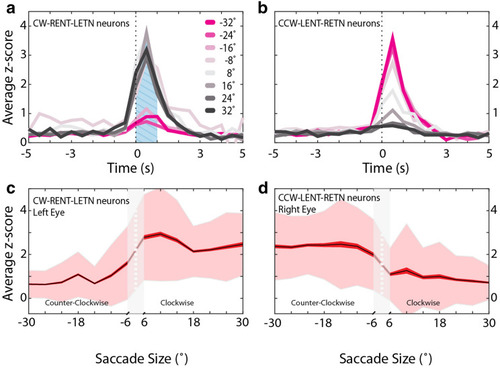
SA neuron tuning to saccade size. ( |
|
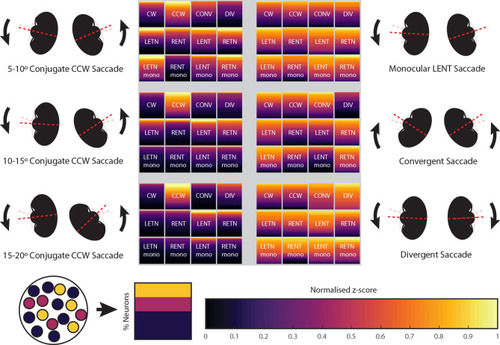
Summary of observed hindbrain saccade-associated activity across different saccade types. Each square refers to the population of neurons listed, except in the case of CW and CCW which refer to CW-LETN-RENT and CCW-LENT-RETN type neurons (see Fig. |