- Title
-
Pathways Regulating Establishment and Maintenance of Cardiac Chamber Identity in Zebrafish
- Authors
- Yao, Y., Marra, A.N., Yelon, D.
- Source
- Full text @ J Cardiovasc Dev Dis
|
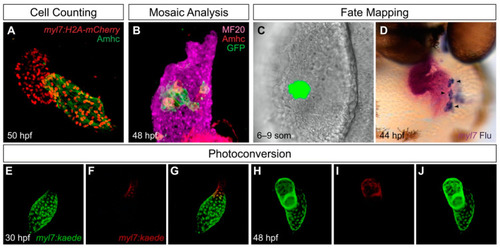
Useful tools for analysis of cardiac chamber development in zebrafish. (A) Transgenes such as Tg(myl7:H2A-mCherry) label myocardial nuclei (red) and facilitate counting of the cardiomyocytes in each chamber; the atrial myosin heavy chain Amhc (green) distinguishes the atrium from the ventricle. Lateral view of wild-type heart at 50 h post-fertilization (hpf) is adapted from [27]. (B) Mosaic analysis enables assessment of the cell-autonomy of gene function. In this example, adapted from [27], mosaic distribution of the transgene Tg(hsp70:dnfgfr1-eGFP) (green) inhibits FGF signaling in specific ventricular cells, several of which exhibit ectopic Amhc, indicating a cell-autonomous requirement for FGF signaling to repress amhc expression in the ventricle (lateral view). Magenta fluorescence labels sarcomeric myosin heavy chain using the monoclonal antibody MF20, and red fluorescence indicates localization of Amhc, using the monoclonal antibody S46. (C,D) Fate mapping follows progenitor cells from their origins to their destinations. In this example, adapted from [28], photoactivation of a caged fluorescein-dextran lineage tracer marks a small group of cells in the anterior lateral plate mesoderm (ALPM) at the 6–9 somite (som) stage (C, dorsal view). Later, labeled progeny of these cells (arrowheads) are found in the atrium; uncaged fluorescein (blue) is detectable within the myl7-expressing myocardium (magenta) of the heart (D, frontal view). (E–J) Photoconvertible proteins facilitate tracking of cells over time. In this example, adapted from [29], cardiomyocytes express the transgene Tg(myl7:kaede), and regionally restricted photoconversion of Kaede at 30 hpf converts its green fluorescence into red fluorescence near the arterial pole of the heart tube (E–G). Later, visualization of retained red fluorescence demonstrates that the labeled cells contribute to the ventricle (H–J). |
|
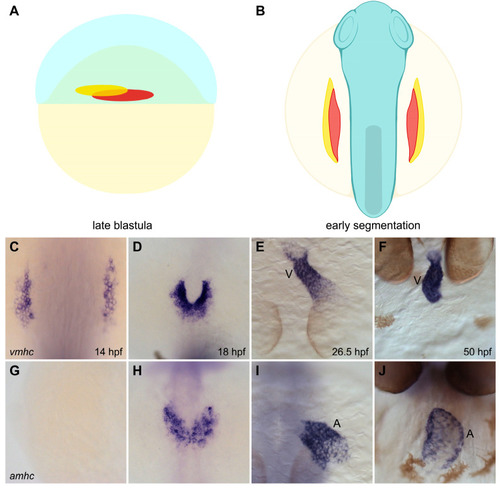
Spatial organization of ventricular and atrial myocardial lineages in the zebrafish embryo. (A,B) Cartoons illustrate the locations of the territories containing ventricular (red) and atrial (yellow) myocardial progenitor cells in the early embryo. Lateral view of the late blastula (A) shows that the regions containing ventricular and atrial myocardial progenitors are spatially organized prior to gastrulation [34]. Dorsal view of the gastrula (B) shows the ALPM regions that contain ventricular and atrial myocardial precursors during early segmentation stages [28]. (C–J) In situ hybridization depicts the expression patterns of vmhc (C–E, dorsal views; F, frontal view) and amhc (G–I, dorsal views; J, frontal view), indicating the relative positions of ventricular and atrial cardiomyocytes as they differentiate and form the heart. Ventricular cardiomyocytes initiate vmhc expression around 14 hpf (C), whereas atrial cardiomyocytes initiate amhc expression around 18 hpf (H) [38,39]; at these stages, ventricular cardiomyocytes are located more medially than atrial cardiomyocytes (D,H). Ventricular and atrial cardiomyocytes go on to occupy separate portions of the heart tube (E,I); later, the ventricular and atrial chambers become morphologically distinct (F,J). V, ventricle, A, atrium. Images adapted from [27]; illustrations by Jessyka T. Diaz. |
|
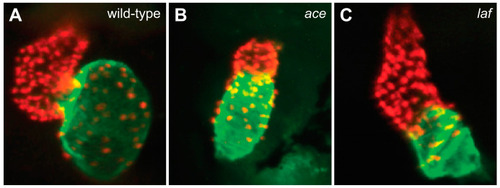
FGF and BMP signaling promote ventricular and atrial cardiomyocyte formation, respectively. Compared to the chambers of the wild-type heart at 48 hpf (A), the ace (fgf8a) mutant heart (B) exhibits a substantially reduced ventricle, and the laf (acvr1l) mutant heart (C) exhibits a substantially reduced atrium. Red fluorescence labels cardiomyocyte nuclei, and green fluorescence indicates localization of Amhc. Images adapted from [54,59]. |
|
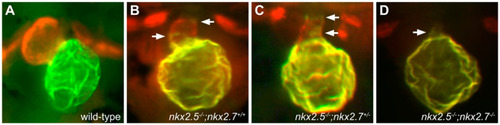
nkx genes are required for maintenance of ventricular cardiomyocyte identity. Compared to the wild-type heart at 52 hpf (A), nkx2.5 mutants (B) exhibit a reduced ventricle and an enlarged atrium, with ectopic Amhc in some ventricular cells (arrows), and additional loss of a single allele of nkx2.7 (C) leads to increased presence of ectopic Amhc in the ventricle (arrows). nkx2.5;nkx2.7 double homozygotes (D) exhibit only a small ventricular remnant, with Amhc present throughout (arrow). Red fluorescence labels sarcomeric myosin heavy chain, and green fluorescence indicates localization of Amhc. Images adapted from [29]. |
|
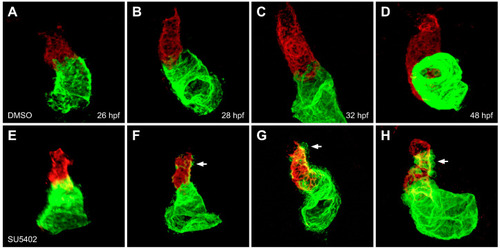
FGF signaling is required to maintain ventricular cardiomyocyte identity. In contrast to control embryos treated with DMSO (A–D), embryos treated with SU5402 beginning at 18 hpf (E–H) exhibit gradual accumulation of ectopic Amhc (arrows), accompanied by diminishing levels of Vmhc in the ventricle, between 28 and 48 hpf (F–H). Red fluorescence indicates localization of Vmhc, and green fluorescence indicates localization of Amhc. Images adapted from [27]. |