- Title
-
Hyaloid vasculature and mmp2 activity play a role during optic fissure fusion in zebrafish
- Authors
- Weaver, M.L., Piedade, W.P., Meshram, N.N., Famulski, J.K.
- Source
- Full text @ Sci. Rep.
|
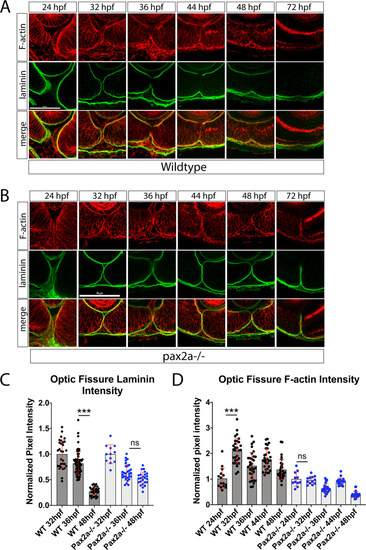
An increase in F-actin dynamics preceding laminin degradation during optic fissure fusion is disrupted in pax2a−/− embryos. ( |
|
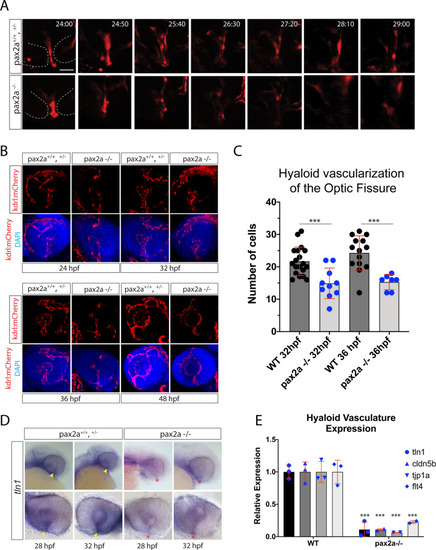
Pax2a is necessary for recruitment of hyaloid vasculature into the optic fissure. ( |
|
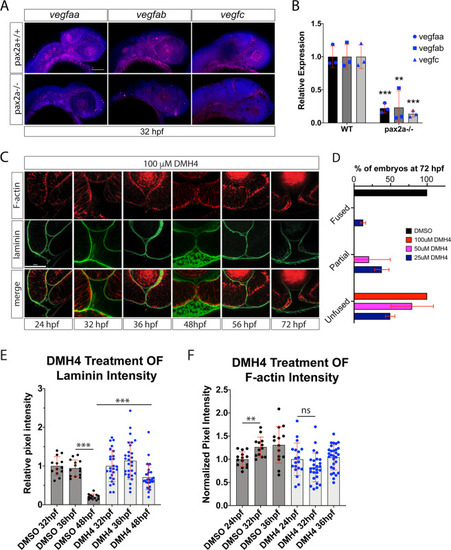
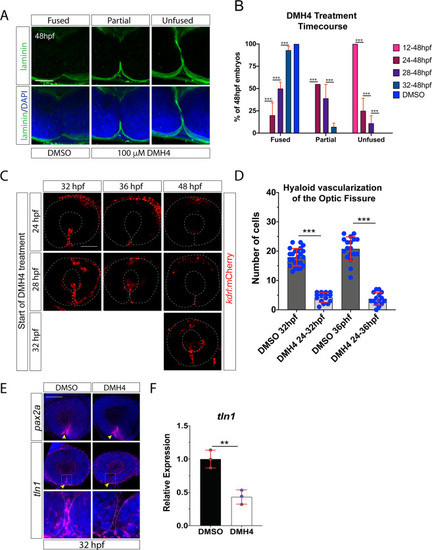
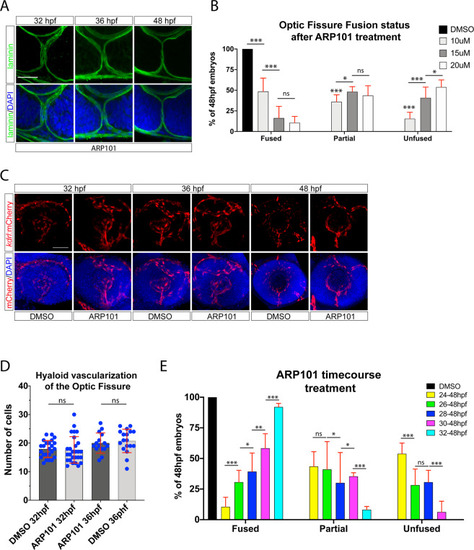
Inhibiting angiogenesis disrupts optic fissure fusion mechanics. ( EXPRESSION / LABELING:
PHENOTYPE:
|
|
VEGF signaling is plays a role in optic fissure fusion. ( |
|
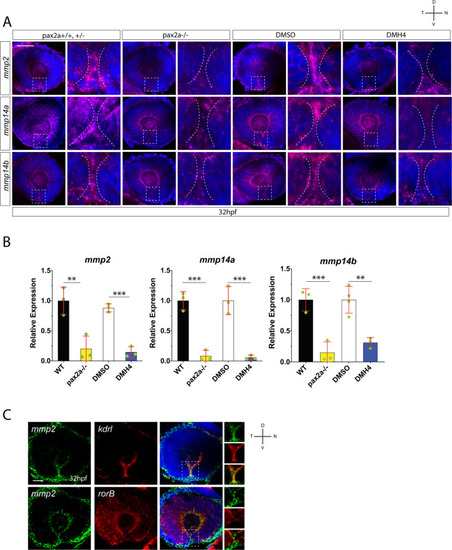
Hyaloid vasculature is a source of mmp2 during optic fissure fusion. ( |
|
Proper timing of mmp2 activity is required for optic fissure fusion. ( PHENOTYPE:
|