- Title
-
Ethyl Acetate Fraction of Aqueous Extract of Lentinula edodes Inhibits Osteoclastogenesis by Suppressing NFATc1 Expression
- Authors
- Lee, H., Lee, K., Lee, S., Lee, J., Jeong, W.T., Lim, H.B., Hyun, T.K., Yi, S.J., Kim, K.
- Source
- Full text @ Int. J. Mol. Sci.
|
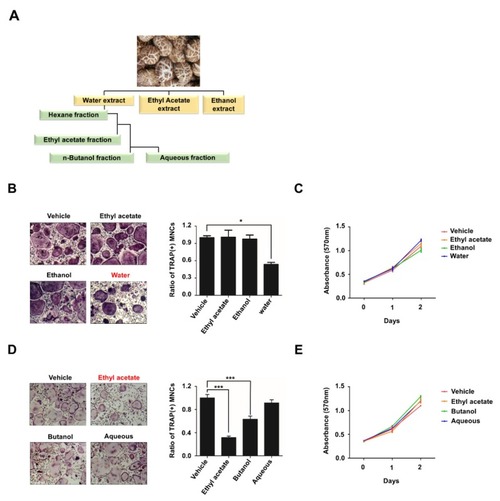
Ethyl acetate fraction of aqueous extract of |
|
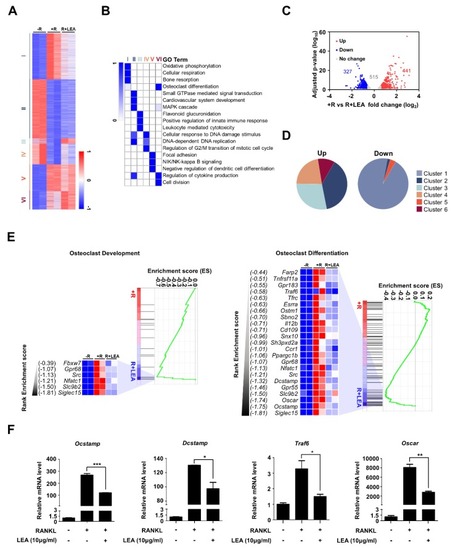
LEA alters gene expression profiling in BMMs. ( |
|
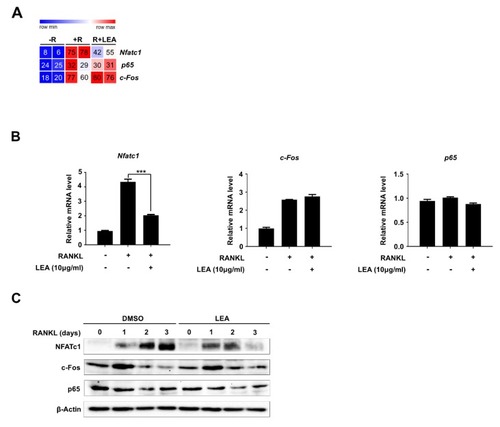
LEA represses RANKL-mediated NFATc1 expression. ( |
|
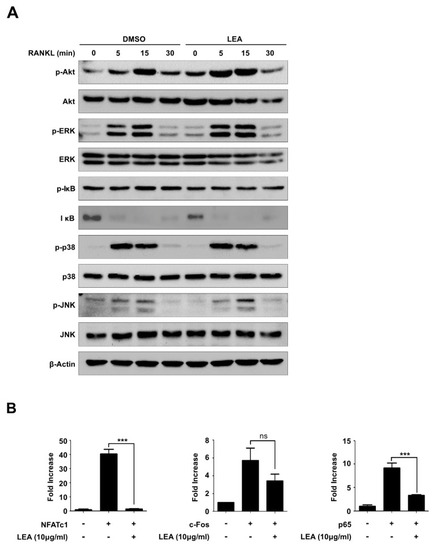
LEA suppresses NFATc1 expression by blocking both NF-κB and NFATc1-mediated transactivity. ( |
|
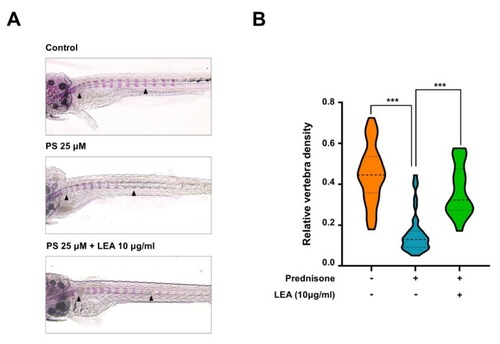
Anti-osteoporotic effect of LEA in a model of prednisolone-induced osteoporosis in zebrafish. ( PHENOTYPE:
|