- Title
-
Intraperitoneal dye injection method for visualizing the functioning lymphatic vascular system in zebrafish and medaka
- Authors
- Saito, E., Isogai, S., Deguchi, T., Ishida, K., Nozaki, T., Ishiyama, E., Wayama, M., Shimoda, H.
- Source
- Full text @ Dev. Dyn.
|
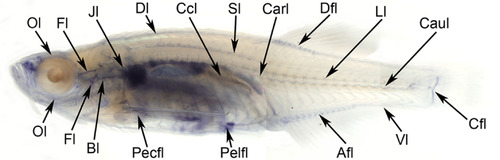
Lymphatic collecting ducts of adult zebrafish (crystal). Intraperitoneal trypan blue injection allowed visualization of all lymphatic ducts of the teleost according to Kampmier.21 To present every lymphatic duct precisely, a fixed specimen was cleared with pure glycerol. Abbreviations (see Table 1) |
|
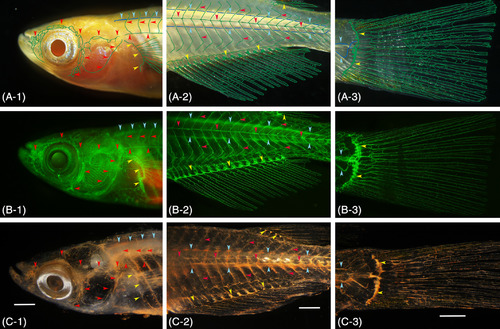
Gross anatomical identification of the lymphatic vascular system in pFLT4‐EGFP transgenic medaka (adult). A, Conventional stereomicroscopy image of live fish. Superficial (green) and deep (blue) lymphatic ducts and the lymphatic ducts of an appendage (green) are diagrammatically delineated. B, Fluorescence light microscopy image of the same live fish. C, Conventional stereomicroscopy image of the same fish 24 hours after the intraperitoneal cinnabar dye injection. All of the functional lymphatic vascular system was labeled with cinnabar. To verify parietal lymphatic ducts precisely, the brain, gills, and visceral organs were removed from the fixed fish, and the specimen was then cleared with pure glycerol. A‐1, B‐1, C‐1, superficial lymphatic ducts of the cranial region (ie, facial, jugular, opercular, and orbital) and of the trunk (ie, lateral with metameric dorsal and ventral tributaries) are indicated by red arrowheads. Blue and yellow arrowheads indicate the deep lymphatic duct (ie, spinal) and lymphatic vascular system of the pectoral fin on the trunk. A‐2, B‐2, C‐2, the superficial lymphatic system of the trunk and tail (ie, lateral) is indicated by red arrowheads. Blue and yellow arrowheads indicate the deep lymphatic duct (ie, spinal and cardinal‐caudal) and lymphatic vascular systems of the fin (anal and dorsal). A‐3, B‐3, C‐3, lymphatic vascular system of the caudal fin. Blue and yellow arrowheads indicate the deep lymphatic ducts (ie, spinal and caudal) and proximal lymphatic duct of the caudal fin. White bar; 1 mm |
|
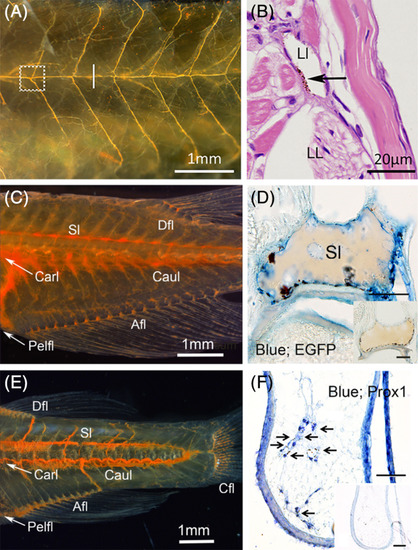
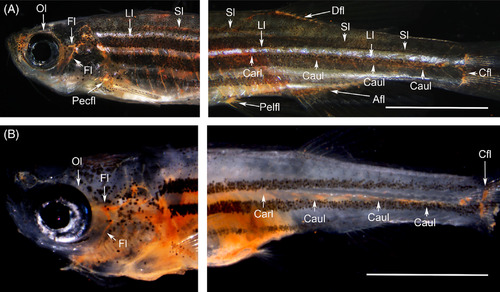
Histological identification of lymphatic ducts labeled with cinnabar. A, Lateral lymphatic duct system of the pflt4‐EGFP adult medaka (left; cranial and top; dorsal). Subcutaneous lymphatic duct was labeled with cinnabar dye in the left trunk. B, Magnified cross‐section (hematoxylin‐eosin) of the duct labeled with cinnabar particles (arrow) at the level indicated by the white bar in Figure 3A (right; lateral and top; dorsal, LL, lateral line; Ll, lateral lymphatic duct). C, Lymphatic ducts in the trunk and tail of the pflt4‐EGFP adult medaka (left; cranial and top; dorsal). Deep lymphatic ducts (Carl‐Caul, cardinal‐caudal lymphatic; Sl, spinal lymphatic) and lymphatic vascular system in appendages (Afl, Anal fin lymphatic; Dfl, dorsal fin lymphatic; Pefl, pelvic fin lymphatic) were labeled with cinnabar dye. D, Cinnabar particles (blackish pigments) adhered to the EGFP‐immunopositive endothelia lining of the spinal lymphatic duct (blue) in the cross‐section of pflt4‐EGFP medaka. E, Lymphatic ducts in the trunk and tail of the adult zebrafish (left; cranial and top; dorsal). Deep lymphatic ducts (Carl‐Caul, cardinal‐caudal lymphatic; Sl, spinal lymphatic) and lymphatic vascular systems in appendages (Afl, Anal fin lymphatic; Cfl, caudal fin lymphatic), Dfl, dorsal fin lymphatic; Pefl, pelvic fin lymphatic) were labeled with cinnabar dye. F, Prox1 immunostaining for transversely sectioned lymphatic vessels in the pelvic fin of cinnabar‐injected zebrafish. Cinnabar particles (arrows) accumulated around the Prox1‐immunopositive vessels (blue). Histochemical controls in respective sections adjacent to D,F. Bars, 50 μm |
|
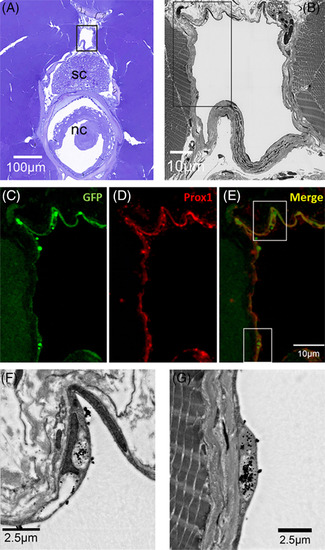
Correlative scanning microscopy showing cinnabar accumulation in Prox1‐ and EGFP‐immunopositive endothelia of the spinal lymphatic duct in pFLT4‐EGFP adult medaka. A, Cross‐section (toluidine blue) of the trunk to show the relative position of the spinal lymphatic duct (in black frame) in relation to the spinal cord (sc) and notochord (nc). B, Dark‐field SEM image of the spinal lymphatic duct in the frame of Figure 4A. C‐E, Immunostained lymphatic endothelia lining the spinal lymphatic duct in the frame of Figure 4B. C‐E show GFP (green), Prox1 (red), and GFP+ Prox1 (merge) expression, respectively. F,G, Dark‐field SEM images of lymphatic endothelium in the frames of Figure 4E. Note the dense black particles (cinnabar) in/on the endothelium |
|
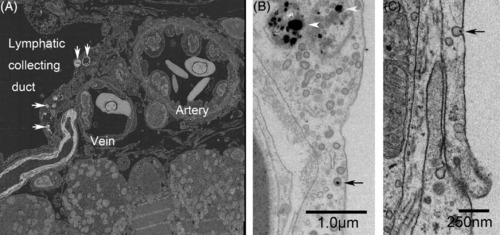
Lateral lymphatic duct labeled with cinnabar (HgS) and collaterally located subcutaneous artery and vein in the trunk of adult zebrafish. A, Back‐scatter image from the reflection transmission electron microscope revealed that electron‐dense particles (cinnabar; white arrows) accumulated in the endothelia lining the lateral lymphatic duct but not in those of the arteries or veins. B and C, In higher‐magnification views of the lymphatic endothelium, accumulation of dense particles can be seen in the lysosomes (white arrowheads), and a single particle (black arrow) is associated with a small pit (or caveola) and a coated pit |
|
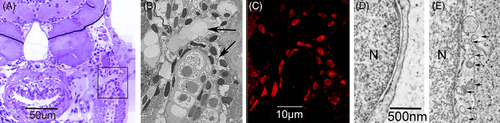
Cytoplasmic differentiation related to the maturation of the lymphatic endothelium in the zebrafish embryo. At the lymph‐sac of the jugular angle, venous progenitor cells begin to differentiate into functional lymphatic endothelium at 2 dpf and become functional at 9 dpf. A, Cross‐section (toluidine blue) of the trunk to show the lymph‐sac (in black frame) at the jugular angle of the 5 dpf zebrafish. B, Dark‐field SEM image of the lymph‐sac (black arrows) in the frame of Figure 7A. C, Correlative prox1‐immunopositive nuclei of the endothelium lining the lymph‐sac in the frame of Figure 7A. D, TEM images of progenitor cells at 5 dpf. E, The functional lymphatic endothelium at 9 dpf revealed that small pits (or caveola) and coated pits (black arrows) emerged in the cytoplasm of lymph‐progenitor cells after 5 dpf and rapidly increased in number in the functional lymph endothelium. N, nucleus |
|
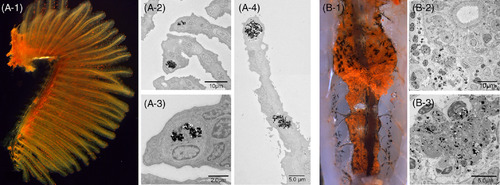
Cinnabar dye in the gills (A‐1, left lateral view) and kidney (B‐1, ventral view) of adult zebrafish. The cinnabar dye predominantly accumulated in the gills and kidney, but the distribution pattern was easily distinguishable from that of lymphatic vessels by macroscopic visual examination. While the dye was distributed diffusely in the lymphatic ducts, it was identified as scattered red spots or granules in the gills (A‐1) and kidney (B‐1). As shown in the TEM images, monocyte/macrophage‐like cells took up the cinnabar particles in the secondary branchial lamella (A‐2,3,4) and in the blood‐forming tissue of the mesonephros (B‐2,3) |
|
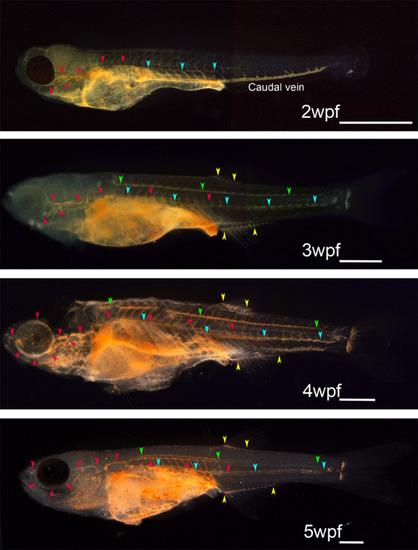
Developmental atlas of the functioning lymphatic vascular system in zebrafish obtained using intraperitoneal dye injection. Every functional lymphatic duct exhibits lymph flow and the ability to take up foreign particles in zebrafish. The functioning lymphatic vascular system was visualized by intraperitoneal dye injection in zebrafish at 2 to 5 wpf. Red arrowheads indicate superficial lymphatic ducts in the cranial region (facial and orbital lymphatics) and in the trunk (lateral lymphatic). Blue and green arrowheads indicate deep lymphatic ducts (cardinal‐caudal and spinal lymphatic, respectively). Yellow arrowheads indicate anal and dorsal fin lymphatics. Primitive caudal vein took up cinnabar particles actively during early developmental stages as shown in 2wpf zebrafish, but the phagocytotic character of the caudal vein was lost as embryonic lymphatic vascular system become functional. White bars; 1 mm |
|
Functional lymph vascular system in the flt4 C526Δ (officially named flt4 um159) adult zebrafish mutant visualized through intraperitoneal dye injection (Abbreviations; see Table 1). A, Although subcutaneous color pigments interfered with visualization, all the functional lymph ducts were labeled with cinnabar in the wild‐type zebrafish (adult). B, Cinnabar dye labeled the branchial, facial and orbital lymph ducts in the cranial region but only weakly labeled the cardinal (thoracic duct) and caudal lymph vessels in the trunk and tail of the flt4 um159 mutant (3 months postfertilization). To verify each parietal lymphatic duct, the brain, gills, and visceral organs were removed from the wild‐type fish (A), but the gills and visceral organs remained in the mutant fish (B). Both fixed specimens were cleared with pure glycerol. White bars; 5 mm |