- Title
-
Emergence of consistent intra-individual locomotor patterns during zebrafish development
- Authors
- Fitzgerald, J.A., Kirla, K.T., Zinner, C.P., Vom Berg, C.M.
- Source
- Full text @ Sci. Rep.
|
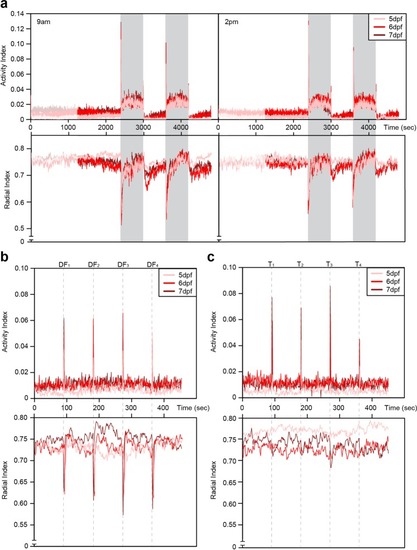
Time series plots from behavior experiments. ( |
|
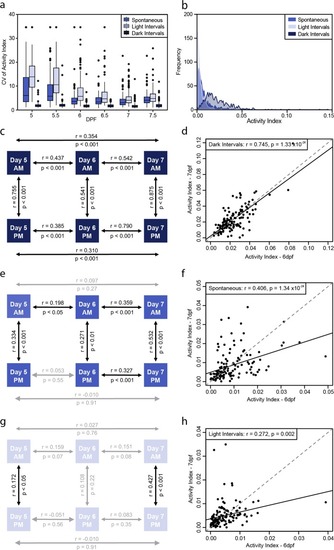
Behavioral intra-individual variability in a population of 132 larvae for the activity index. ( |
|
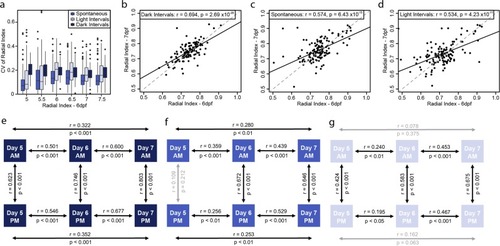
Behavioral intra-individual variability in a population of 132 larvae for the radial index. ( |
|
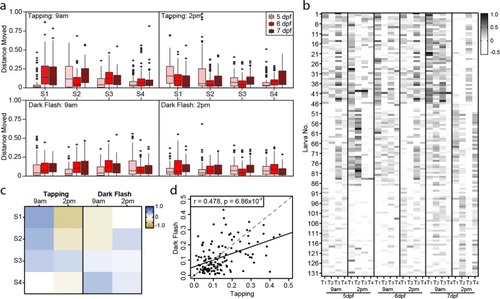
Individual responses to startle stimulus. ( |
|
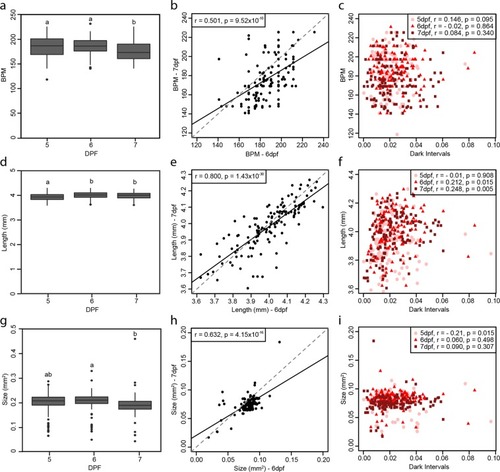
Physiology and morphometric parameter comparisons. Boxplots representing the average measure of all 132 larvae of ( |