- Title
-
CFTR mutation enhances Dishevelled degradation and results in impairment of Wnt-dependent hematopoiesis
- Authors
- Sun, H., Wang, Y., Zhang, J., Chen, Y., Liu, Y., Lin, Z., Liu, M., Sheng, K., Liao, H., Tsang, K.S., Zhang, X., Jiang, X., Xu, W., Mao, M., Chan, H.C.
- Source
- Full text @ Cell Death Dis.
|
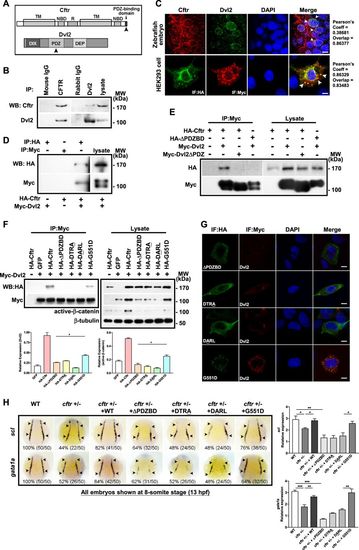
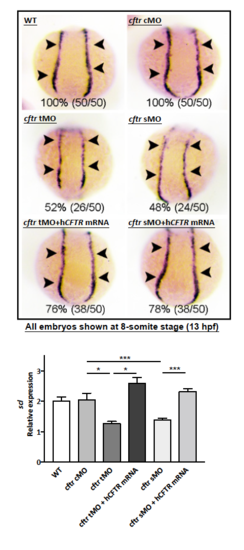
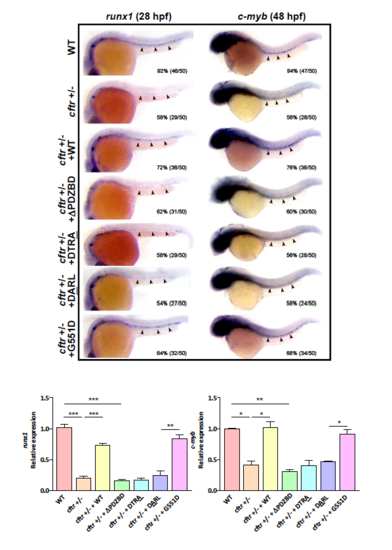
Cftr PDZBD but not its channel function is required for its interaction with Dvl2 and hematopoiesis a Schematic drawing of PDZBD in Cftr and PDZ domain in Dvl2. Referring to Gee, H. Y. (2011)[57] and Wallingford, J. B. (2005)[58]. NBD nucleotide-binding domain, TM transmembrane domain, R regulatory domain, DIX Dishevelled and AXIN domain, DEP Dishevelled EGL-10 Pleckstrin domain. b Co-immunoprecipitation (Co-IP) of endogenous Dvl2 and Cftr in zebrafish embryos. c Co-localization of Cftr and Dvl2 in zebrafish embryo and HEK293 cells. Arrowheads indicate co-localized sites. Scale bar 5 μm. d In vitro binding assay identifies the physical interaction of Cftr with Dvl2. e Co-IP of exogenous Cftr and Dvl2 in HEK293 cells showing lack of interaction when Cftr PDZBD or Dvl2 PDZ domain is deleted. f Co-IP showing the interaction of Dvl2 with G551D, but not Cftr PDZBD mutants (either deletion or point mutation), and western blotting showing that Cftr and G551D overexpression increased the expression of Dvl2 and active-β-catenin more significantly than that induced by Cftr PDZBD mutants. g Immunofluorescence analysis in HEK293 cells shows that either of the CFTR PDZBD mutants is not co-localized with Dvl2, whereas overexpressed G551D is co-localized with Dvl2. h Effects of different cftr mutants mRNA in rescuing hematopoietic defects in cftr mutant zebrafish embryos. Injection of cftr PDZBD mutants individually, with deletion or point mutation, could not rescue the hematopoietic defect in cftr mutant embryos. Injection of G551D rescues the hematopoietic defect in cftr mutant embryos. Embryos shown are dorsal views with anterior to the top at 8-somite stage (13 hpf) EXPRESSION / LABELING:
PHENOTYPE:
|
|
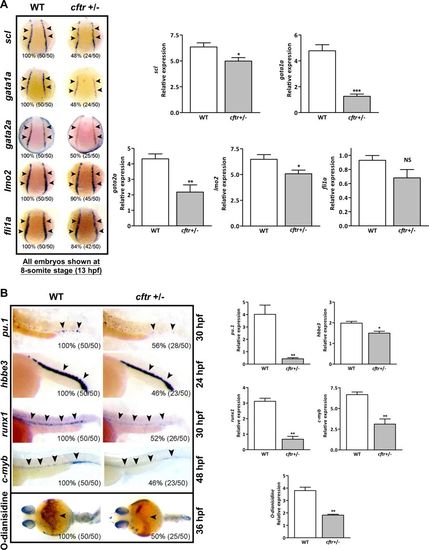
Cftr critically regulates hematopoiesis in zebrafish. a cftr mutants show hematopoietic defects at 8-somite stage (13 hpf). Embryos shown are dorsal views with anterior oriented at the top. b cftr mutants show hematopoietic defects at later stages. Embryos shown are lateral views with anterior to the left. Bottom panel: cftr mutants displayed decreased hemoglobin staining by O-dianisidine. Embryos shown are ventral views with anterior to the left. Arrowheads indicate the expression sites of each marker gene. All genes were assayed by WISH. Histogram representing the relative expression detected by signal strength grayscale using software ImageJ at corresponding assay. The percentage and numbers indicated in each picture are the ratio for the number (left in bracket) of affected embryos with phenotype similar to what is shown in the picture and the total number (right in bracket) of observed embryos. The same number labeling was used thereafter EXPRESSION / LABELING:
PHENOTYPE:
|
|
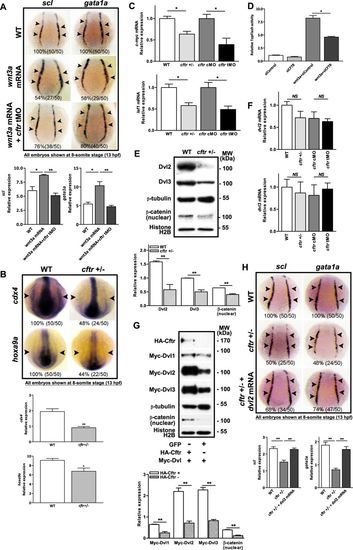
Involvement of Cftr in Wnt-dependent hematopoiesis in zebrafish. WISH showing impaired wnt3a-induced expression of scl and gata1a in wnt3a mRNA and cftr morpholino co-injected zebrafish (a) and decreased expression of cdx4 and hoxa9a in cftr mutants (b). c Quantitative RT-PCR showing significantly reduced c-myc and lef1 mRNA levels in cftr mutants. d CFTR knockdown by siRNA attenuated the Wnt reporter TopFlash activity in HEK293 cells (n = 3). e Western blot analysis showing reduced Dvl2, 3 and nuclear β-catenin expression in cftr mutants. f Quantitative RT-PCR showing no significant changes in dvl2, 3 mRNA levels in cftr mutants. g Western blot analysis showing increased Dvl1-3 protein levels in co-expressing Cftr with Dvl1-3 HEK293 cells as compared to Dvl1-3 alone controls. h Effects of dvl2 mRNA in rescuing hematopoietic defects in cftr mutants. Embryos shown in a, b, and h are dorsal views with anterior to the top at 8-somite stage (13 hpf) EXPRESSION / LABELING:
PHENOTYPE:
|
|
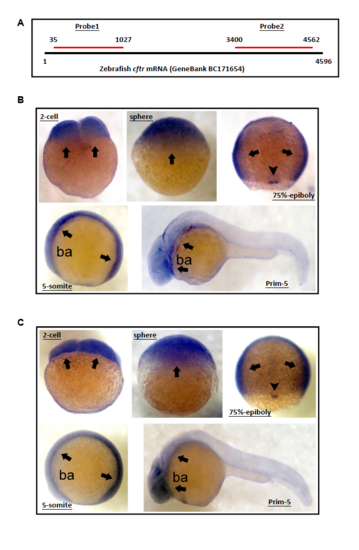
Spatiotemporal expression pattern of cftr transcript in zebrafish embryos. |
|
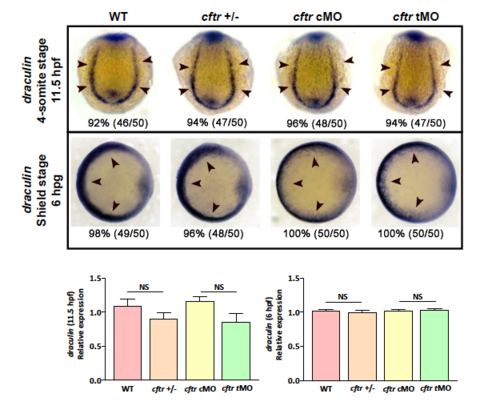
Lateral mesoderm marker draculin expression showing insignificant change in cftr mutant and morphants. EXPRESSION / LABELING:
|
|
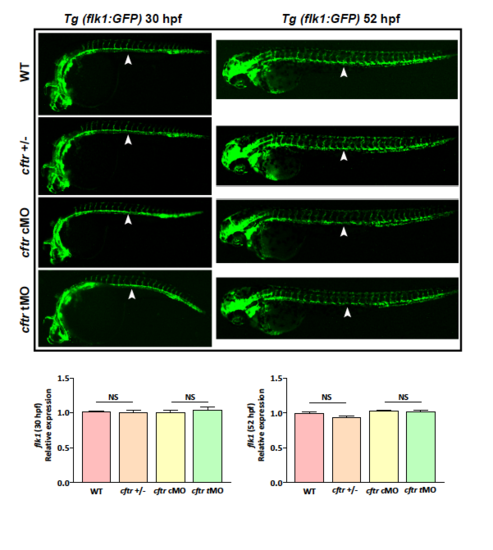
Vascular endothelium marker flk1 expression showing insignificant change in cftr mutant and morphants. |
|
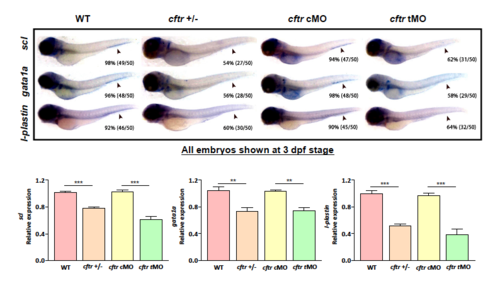
The expression of definitive hematopoietic marker genes decreases in cftr mutant and morphants at 3 dpf. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Specificity of cftr morpholinos on hematopoiesis during zebrafish early embryogenesis. EXPRESSION / LABELING:
PHENOTYPE:
|
|
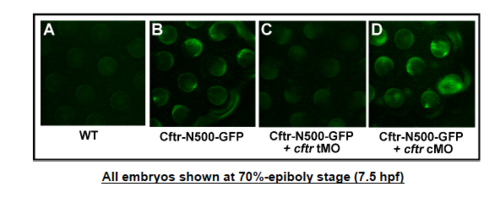
Effectiveness of cftr translation-blocker morpholino (cftr tMO). |
|
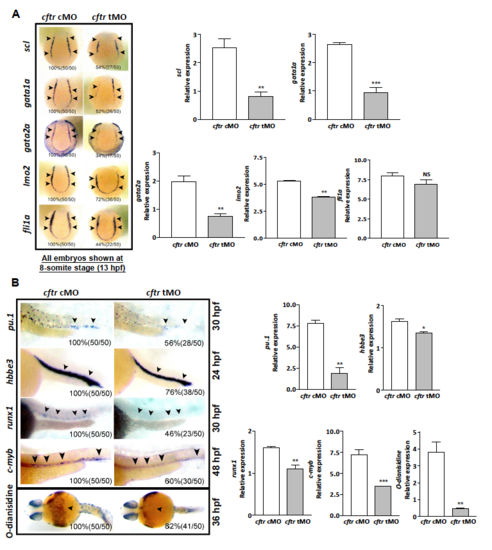
Hematopoietic marker genes expression decreases in cftr morphants. EXPRESSION / LABELING:
PHENOTYPE:
|
|
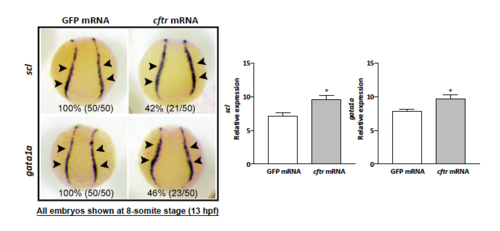
Overexpression of Cftr enhances hematopoietic marker genes expression in zebrafish. |
|
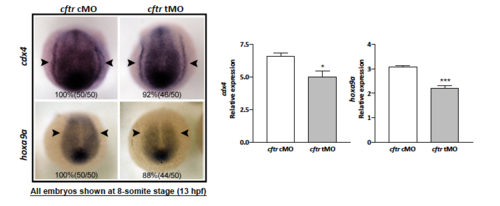
Decreased expression of Wnt target gene cdx4 and hoxa9a in cftr morphants. EXPRESSION / LABELING:
PHENOTYPE:
|
|
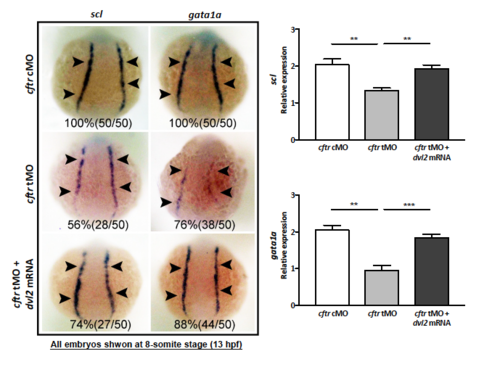
dvl2 mRNA rescues the hematopoietic defect in cftr morphants. EXPRESSION / LABELING:
PHENOTYPE:
|
|
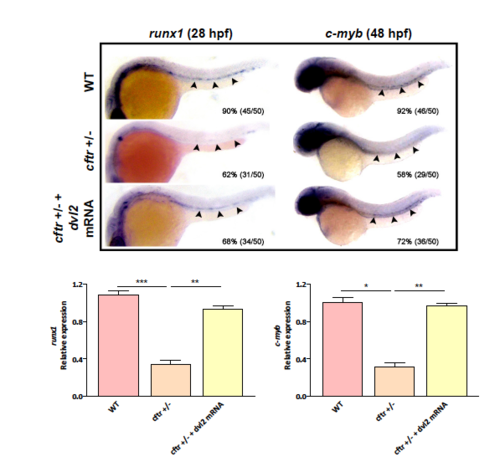
Effects of dvl2 mRNA in rescuing definitive hematopoietic defects in cftr mutants. |
|
Effects of different cftr mutants mRNA in rescuing definitive hematopoietic defects in cftr mutant zebrafish embryos. |