- Title
-
CD146 is required for VEGF-C-induced lymphatic sprouting during lymphangiogenesis
- Authors
- Yan, H., Zhang, C., Wang, Z., Tu, T., Duan, H., Luo, Y., Feng, J., Liu, F., Yan, X.
- Source
- Full text @ Sci. Rep.
|
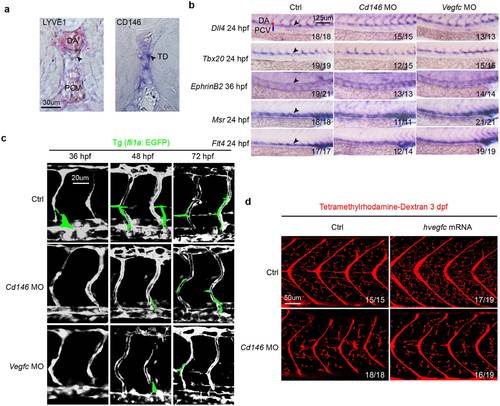
CD146 modulates VEGF-C induced lymphatic development in zebrafish. (a) CD146 expression in the zebrafish embryos at 5 dpf was detected by whole-mount in situ hybridization (WISH). lyve1b was used as positive control. Black arrows indicate the position of TD. DA, dorsal aorta; PCV, posterior cardinal vein; and TD, thoracic duct. Scale bars: 30 μm. (b) The effect of knockdown cd146 or vegfc on blood vessels. Bright-field lateral views of the trunk of embryos injected with control, cd146 or vegfc specific MOs. Embryos were stained by WISH for a panel of arterial markers of dll4, tbx20 and ephrinB2 and venous markers of msr and flt4. Black arrows, blood vessels; DA, dorsal aorta; and PCV, posterior cardinal vein. Scale bars: 125 μm. (c) The effect of knockdown cd146 or vegfc on vasculature of blood and lymph. The control, cd146 or vegfc specific MO were injected into the transgenic zebrafish Tg (fli1a: EGFP) embryos, respectively. The embryos harvested at 36 hpf, 48 hpf and 72 hpf were analyzed. The confocal images were flanked by redrawing of the vessel contours. Transient lymphangiogenic structures (lymphangiogenic sprouts; PL cells) were labeled with light green. The other vessels (PCV, DA and ISV) were labeled with dark grey. Scale bars: 20 μm. (d) The effects of cd146 knockdown on development of lymphatic capillary. The control or cd146 specific MO was co-injected with control or human vegfc mRNA into the transgenic zebrafish Tg (fli1a: EGFP) embryos. Late-phase microangiography was used to visualize lymphatic capillaries in embryos at 3 dpf. Images showed the representative phenotypes of each group and the ratio indicated the embryos with such representative phenotype over the tested embryos. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
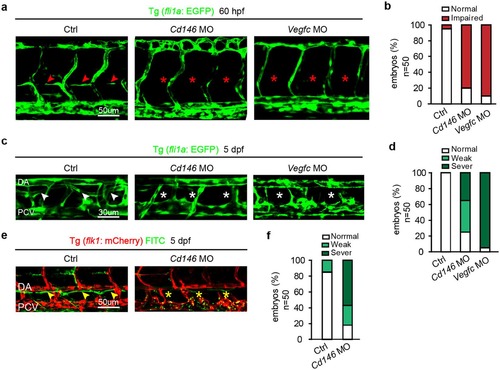
Knockdown of CD146 disrupts lymphangiogenesis in zebrafish. (a,b) The effects of cd146 or vegfc knockdown on PL formation. Tg (fli1a: EGFP) embryos, injected with control, cd146 or vegfc specific MO, were harvested at 60 hpf. Confocal images showed normal PL (red arrows) and impaired PL (red stars) in (a). Scale bars: 50 μm. The percentages of embryos with normal PL cells (normal) and impaired PL string formation (impaired PLs) were shown in (b). (c,d) The effects of cd146 or vegfc knockdown on TD formation. Tg (fli1a: EGFP) embryos, injected with control, cd146 or vegfc specific MO, were harvested at 5 dpf. Confocal images showed normal TD (white arrows) and abnormal TD (white stars) in (c). Scale bars: 30 μm. The percentages of embryos with different phenotypes of TD formation were shown in (d). Relative to the normal embryos, the TD length decreased to 30–90% were classified as weak, and those decreased to 10–30% were classified as severe. (e,f) The effects of cd146 knockdown on TD function. Tg (flk1: mcherry) zebrafish embryos injected with control or cd146 specific MO and FITC-dextran was injected subcutaneously in the tail of those fishes. The embryos were harvested at 5 dpf. Confocal images showed functional TD (yellow arrows) and dysfunctional TD (yellow stars) in (e). Scale bars: 50 μm. The percentages of embryos with different phenotypes of TD formation were shown in (f). The criterion used to classify the TD formation was the same as that used in (d). In all panels, the head of the embryo faces left and dorsal is up. |
|
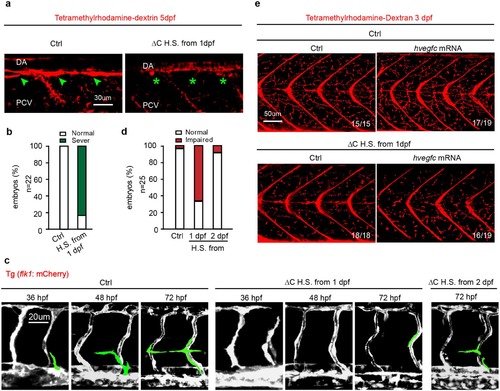
CD146 is essential for lymphangiogenesis independent of angiogenesis. (a,b) Tg (fli1a: EGFP) embryos injected with plasmid of CD146 ∆C-GFP or its empty control, which was induced to express CD146 ∆C-GFP by 37 °C heat-shocking for 30 min. The embryos were harvested at 5 dpf. Tetramethylrhodamine-dextran was used to visualize normal TD (green arrow) and abnormal TD (green stars). The criterion used to classify the TD formation in (b) was the same as that used in Fig. 4d. Scale bars: 30 μm. (c,d) Tg (flk1: mcherry) embryos were treated as same as in (a). The embryos were harvested at 36 hpf, 48 hpf and 72 hpf, respectively. Vasculature of blood and lymphatic vessels were observed by confocal microscopy. Images were flanked by redrawing of the vessel contours. Transient lymphangiogenic structures (lymphangionenic sprouts; PL cells) were labeled light green and the other vessels (PCV, DA and ISV) were labeled dark grey. Scale bars: 20 μm. (e) Tg (fli1a: EGFP) embryos were injected with plasmid of CD146 ∆C-GFP or its control plasmid together with control or human vegfc mRNA and were heat-shocked to express CD146 ∆C-GFP at 37 °C for 30 min. Late-phase microangiography was used to visualize typical phenotype of lymphatic capillaries in embryos at 3 dpf. The typical ratio was showed in each panel. Scale bars: 50 μm. |
|
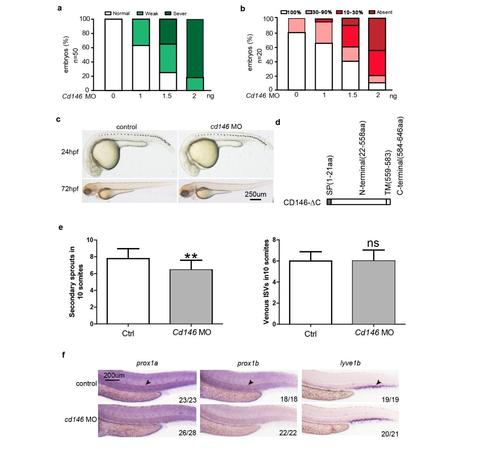
Knockdown of CD146 disrupts PL and TD formation in zebrafish. (a) Quantification of the TD formation defects after injection of different dose of cd146 MO at 5 dpf. Relative to the normal embryos, the TD length decreased to 30%-90% were classified as weak, and those decreased to 10%-30% were classified as sever. (b) Quantification of the PL formation defects after injection of different dose of cd146 MO at 60 hpf. Percentages of embryos displaying complete lack of PL, PL formation over 10%-30% or 30%-90% of its normal length, and a normal PL were represented for each treatment group. (c) The effect of knockdown cd146 on morphology of zebrafish embryo at 24hpf and 72hpf. Bright-field side views of the whole embryos injected with control or cd146 specific MO. Scale bars: 250 μm. (d) Diagrammatic representation of C-terminal domain deleted CD146. (e) Quantificaiton of the number of unilateral secondary sprouts (left) and the fraction of venous ISVs, identified by their connection to the PCV upon confocal screening. **, P<0.05, compared with control group. (f) The effect of knockdown cd146 on lymphatic vessels. Bright-field side views of the trunk of embryos injected with control or CD146 specific MO. Embryos were stained by WISH for a panel of lymphatic markers of prox1a, prox1b and lyve1b. Black arrows, TD. Scale bars: 200 μm. EXPRESSION / LABELING:
PHENOTYPE:
|