- Title
-
Hipk2 and PP1c Cooperate to Maintain Dvl Protein Levels Required for Wnt Signal Transduction
- Authors
- Shimizu, N., Ishitani, S., Sato, A., Shibuya, H., Ishitani, T.
- Source
- Full text @ Cell Rep.
|
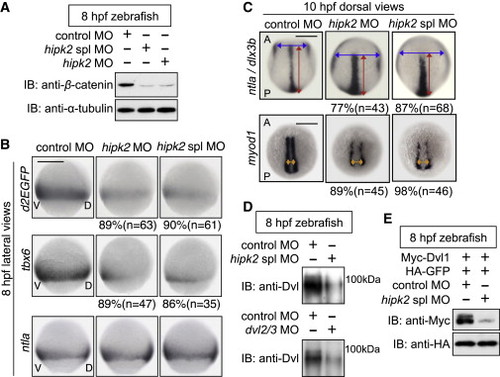
Hipk2 Is Required for Dvl-Mediated Early Embryonic Events in Zebrafish (A) Hipk2 is required for the stabilization of β-catenin in zebrafish. Zebrafish embryos were injected with MOs. Extracts were harvested from embryos at 8 hr postfertilization (hpf) and immunoblotted with anti-zebrafish β-catenin. α-Tubulin was used as a loading control. (B and C) Hipk2 is essential for the β-catenin pathway-mediated posterior mesoderm formation and convergent extension (CE). Embryos were injected with MOs. Panels in (B) show whole-mount in situ hybridization of d2EGFP, tbx6, or ntla in OTM:d2EGFP-transgenic zebrafish embryos. Panels in (C) show whole-mount in situ hybridization of ntla and dlx3b or myod1 in zebrafish embryos. Blue, red, and orange two-way arrows indicate the width of neural plate, the length of notochord, and the width of myod1-expression domain, respectively. The percentages of embryos showing similar expression patterns and total number of MO-injected embryos (n) are shown under each image. In all images, A, P, V, and D indicate anterior, posterior, ventral, and dorsal sides. Scale bar, 200 μm. (D and E) Hipk2 is required for the protein stability of endogenous Dvl (D) and exogenous mouse Dvl1 (E) Dvl in zebrafish. Zebrafish embryos were injected with MOs without (D) or with mouse Myc-Dvl1 and hemagglutinin (HA)-GFP mRNA (E). Extracts were harvested from the embryos at 8 hpf. In (D), embryo extracts were immunoprecipitated and then immunoblotted with anti-Dvl (see te Experimental Procedures for details). In (E), extracts were immunoblotted with indicated antibodies. GFP was used as a loading control. See also Figures S1 and S2. EXPRESSION / LABELING:
PHENOTYPE:
|
|
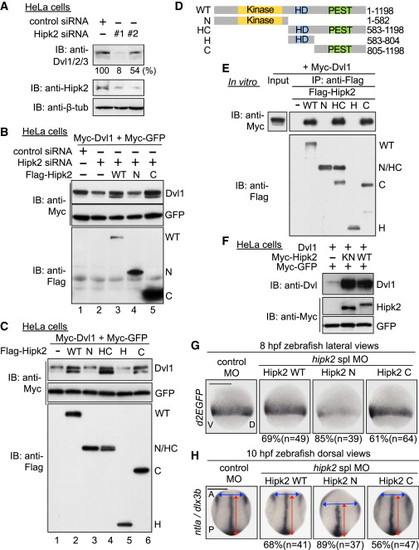
Hipk2 Regulates Dvl Stability in a Kinase Activity-Independent Manner in Mammalian Cells and Zebrafish (A) Hipk2 is required for endogenous Dvl stability in HeLa cells. Cells were treated with either control siRNA or Hipk2 siRNA #1 or #2, and cell extracts were then were immunoblotted with anti-Dvl1/2/3 (which recognizes all types of Dvl), anti-Hipk2, and anti-β-tubulin (7beta;-tub). β-tub was used as a loading control. Relative Dvl protein levels were calculated by determining the ratio of Dvl to β-tub. Values are presented below the top panel as the relative percentage. (B) Hipk2 WT and C, but not Hipk2 N, reversed the Hipk2-knockdown-induced reduction in Dvl expression. HeLa cells were treated with either control siRNA or Hipk2 siRNA#1 and then transfected with Myc-Dvl1 and Myc-tagged GFP (Myc-GFP) with or without Flag-tagged human Hipk2 (Flag-Hipk2) WT, N, and C. (C) Hipk2 increased the Dvl protein levels via its C-terminal domain. HeLa cells were transfected with Myc-Dvl1 and Myc-GFP with empty vector () or Flag-Hipk2 WT, N, HC, H, or C. (D) Schematic diagram of the human Hipk2 deletion mutants. Kinase, kinase domain; HD, homeodomain-interacting domain; PEST, PEST sequence. (E) The C-terminal domain of Hipk2 is essential and sufficient for binding to Dvl. HeLa cells were transfected with empty vector () or Flag-tagged human Hipk2 (Flag-Hipk2) WT, N, HC, H, or C. The cell extracts were mixed with extracts prepared from cells transfected with Myc-Dvl1 and then immunoprecipitated with anti-Flag. Immunoprecipitated complexes were then immunoblotted with indicated antibodies. The expression of Myc-Dvl1 proteins in cell extracts was confirmed by immunoblotting with anti-Myc (“Input” lane). (F) Hipk2 increases the protein level of Dvl1 in a kinase activity-independent manner. HeLa cells were transfected with Dvl1, Myc-tagged mouse Hipk2 (Myc-Hipk2) WT and KN, and Myc-GFP, as indicated. (G and H) Hipk2 regulates the β-catenin pathway-mediated posterior mesoderm formation and CE via its C-terminal domain. Embryos were injected with MOs with or without human Hipk2 WT, N, or C mRNA. Panels show whole-mount in situ hybridization of d2EGFP in OTM:d2EGFP-transgenic zebrafish embryos (G) or ntla and dlx3b in nontransgenic zebrafish embryos (H) fixed at the indicated stages. The percentages of embryos showing similar expression patterns and total number of MO-injected embryos (n) are shown under each image. See also Figures S2 and S3. |
|
Hipk2-PP1c Stabilizes Dvl through Dephosphorylating the Conserved CK1 Sites (A) Amino acid sequence alignment of the C-terminal CK1 phosphorylation regions within vertebrate Dvl proteins. The potential CK1 phosphorylation sites are indicated by red letters. (B) Anti-pDvl1 recognizes the phosphorylation of the conserved CK1 sites on Dvl1 C-terminal region. HeLa cells were transfected with Myc-Dvl1 WT and mutants as indicated. Cell lysates were immunoblotted with indicated antibodies. To clearly show the migration-shift of Dvl proteins, equal quantities of Dvl proteins were resolved. (C) The conserved CK1 sites on Dvl are phosphorylated in zebrafish. Extracts were harvested from the embryos injected with Myc-tagged mouse Dvl1 WT and 3A mRNA at 8 hpf and immunoblotted with indicated antibodies. In (B) and (C), High and low level phosphorylated forms of Dvl are indicated with red and blue arrowheads, respectively. (D) Endogenous Dvl is phosphorylated at the conserved CK1 sites. HeLa cells were treated with DMSO () or 2 nM CalA and 10 μM MG132 for 3 hr. Cell extracts were subjected to immunoprecipitation with anti-Dvl1/2/3 (anti-Dvl). Immunoprecipitates were immunoblotted with indicated antibodies. (E) Hipk2-PP1c dephosphorylates Dvl at the conserved CK1 sites in vivo. HeLa cells were transfected with Myc-Dvl1 WT and 3A and Flag-Hipk2. The cells were then treated with DMSO () or 2 nM CalA and 25 μM MG132 for 3 hr. Cell lysates were immunoblotted with indicated antibodies. (F) Hipk2-PP1c directly dephosphorylates Dvl1 at the conserved CK1 sites. Aliquots of Dvl1 proteins were incubated with or without PP1c and Hipk2 and then immunoblotted with indicated antibodies. In (E) and (F), relative Dvl phosphorylation levels were calculated by determining the ratio of phospho-Dvl1 to total Dvl and these values are presented below the panels as the relative percentages. (G–J) Dvl1 3A is less sensitive to Hipk2 and PP1c than Dvl WT. In (G)–(I), HeLa cells were treated with or without control siRNA, Hipk2 siRNA#2, or PP1c siRNA#1 and then transfected with Myc-Dvl1 WT and 3A, Myc-GFP, and Flag-Hipk2. In (J), zebrafish embryos were injected with MOs with mouse Myc-Dvl1 and HA-GFP mRNA. Extracts were immunoblotted with indicated antibodies. Relative Dvl1 protein levels were calculated by determining the ratio of Dvl1 to GFP. These values are presented below the top panel as the relative percentages. (K–N) Hipk2 regulates the β-catenin pathway through Dvl dephosphorylation in zebrafish. In (K) and (M), classes of phenotypes induced by hipk2 spl MO injection are shown. In (K), class I, weak reduction of OTM:d2EGFP (d2EGFP); class II, strong reduction of d2EGFP. In (M), class I, weak reduction of tbx6; class II, strong reduction of tbx6. In (L) and (N), the distribution of phenotypes in 8 hpf OTM:d2EGFP-transgenic (L) or nontransgenic (N) zebrafish embryos injected with MOs with or without mouse Dvl1 WT or 3A mRNA (40 pg) is shown. n = the total number of MO-injected embryos. See also Figures S5 and S6. |
|
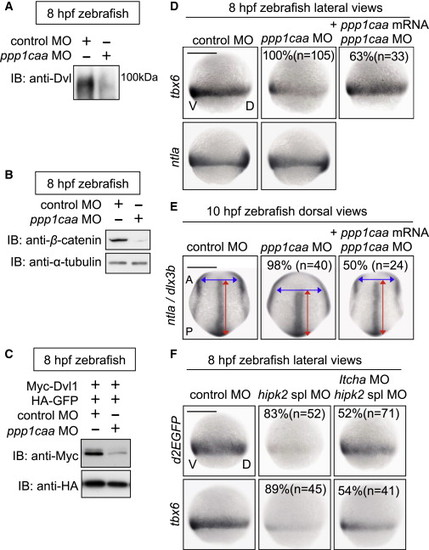
In Zebrafish, PP1c Cooperates with Hipk2 to Sustain Wnt Signal Transduction while Itch Counteracts Hipk2 Activity (A–C) PP1c is required for the protein stability of endogenous Dvl (A) and β-catenin (B) and exogenous mouse Dvl1 (C) in zebrafish. Zebrafish embryos were injected with MOs without (A and B) or with (C) mouse Myc-Dvl1 and HA-GFP mRNA. Extracts were harvested from the embryos at 8 hpf. In (A), embryo extracts were immunoprecipitated and then immunoblotted with anti-Dvl. In (B) and (C), extracts were immunoblotted with indicated antibodies. (D and E) PP1c is required for tbx6 expression and CE. Embryos were injected with control MO or ppp1caa MO (2 ng in D, 4 ng in E) with or without MO-insensitive ppp1caa mRNA. Panels show whole-mount in situ hybridization of tbx6, ntla, or dlx3b in zebrafish embryos. (F) Itch counteracts Hipk2 activity in zebrafish. Embryos were injected with MOs as indicated. Panels show whole-mount in situ hybridization of OTM:d2EGFP (d2EGFP) or tbx6 in OTM:d2EGFP-transgenic (top) or nontransgenic (bottom) zebrafish embryos. In (D)–(F), the percentages of embryos showing similar expression patterns and the total number of MO-injected embryos (n) are shown under each image. EXPRESSION / LABELING:
PHENOTYPE:
|
|
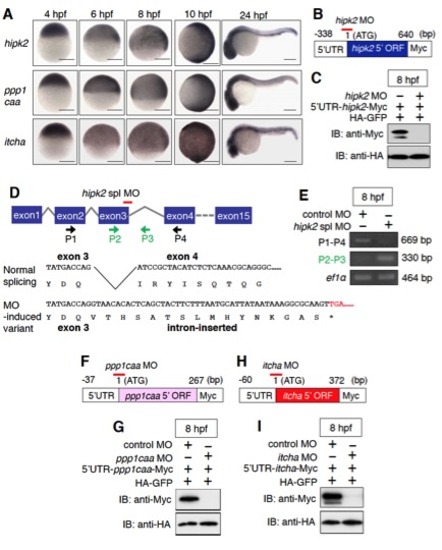
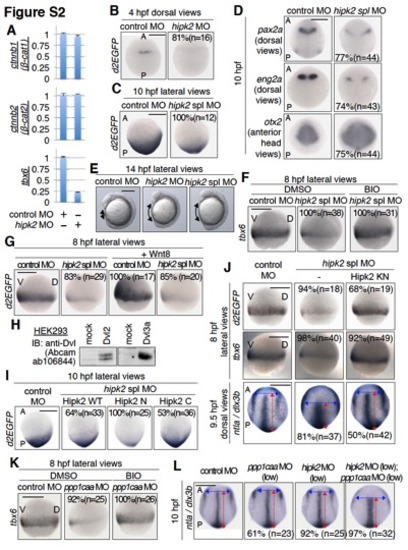
The expression patterns of zebrafish hipk2, ppp1caa, and itch and the effects of hipk2 MOs, ppp1caa MO, and itcha MO. Related to Figure 1. |
|
Hipk2 and PP1c are involved in Dvl–mediated Wnt signaling during zebrafish early embryogenesis. Related to Figures 1 and 2. |