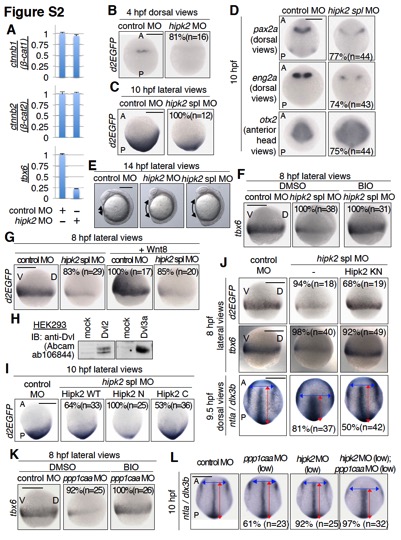
Fig. S2
Hipk2 and PP1c are involved in Dvl–mediated Wnt signaling during zebrafish early embryogenesis. Related to Figures 1 and 2.
(A) Hipk2 is required for the expression of tbx6 mRNA, but not the expression of endogenous β-catenin mRNA in zebrafish. Control MO or hipk2 MO were injected into one cell-stage zebrafish embryos as indicated. At 8 hpf, 30 embryos were collected and total RNA was purified. The relative mRNA expression levels of zebrafish β-catenins, ctnnb1 (β-cat1) and ctnnb2 (β-cat2), and tbx6 were analyzed by quantitative RT-PCR. The mean and standard deviation of triplicate measurements from one of two independent experiments are presented.
(B, C) Hipk2 is required for the activation of Wnt/β-catenin pathway in 4 and 10 hpf zebrafish embryos.
(D) Hipk2 regulates brain anterior-posterior patterning.
(E) hipk2 knockdown embryos failed to extend properly around the yolk at 14 hpf. In (B–E), embryos were injected with control MO, hipk2 MO, or hipk2 spl MO. In (B-D), panels show whole mount in situ hybridization of OTM:d2EGFP (d2EGFP), pax2a, eng2a, or otx2 in OTM:d2EGFP-transgenic (B, C) or non-transgenic (D) zebrafish embryos fixed at the indicated stages. In (E), panels show the lateral views of embryos. A, P, V, and D indicate anterior, posterior, ventral, and dorsal sides. Scale bar = 200 μm. The percentages of embryos showing similar gene expression patterns and total number of MO-injected embryos (n) are shown in each image.
(F, G) Hipk2 appears to function upstream of GSK-3β and downstream of Wnt8. Non-transgenic (F) or OTM:d2EGFP-transgenic (G) zebrafish embryos were injected with control MO or hipk2 spl MO without (F) or with zebrafish Wnt8 mRNA (G), and then untreated (G) or treated with DMSO or 10 μM of the GSK-3β inhibitor BIO from 5 to 8 hpf (F) and fixed at 8 hpf. Panels show whole mount in situ hybridization of otx2 or OTM:d2EGFP (d2EGFP). The percentages of embryos showing similar gene expression patterns and total number of MO-injected embryos (n) are shown in each image. Scale bar = 200 μm. (H) Anti-Dvl antibody recognizes zebrafish Dvl2 and Dvl3a proteins. HEK293 cells were transfected with empty vector (mock) or expression plasmids encoding zebrafish Dvl2 or Dvl3a and cell lysates were immunoblotted with anti-Dvl antibody (Abcam ab106844).
(I) Hipk2 regulates the β-catenin pathway–mediated posterior tissue formation via its C-terminal domain. Embryos were injected with control MO or hipk2 spl MO with or without 40 pg of MO=insensitive human Hipk2 WT, N, or C mRNA, as indicated. Panels show whole mount in situ hybridization of OTM:d2EGFP (d2EGFP) in OTM:d2EGFP-transgenic zebrafish embryos fixed at 10 hpf.
(J) Hipk2 functions in a kinase activity-independent manner in vivo. Embryos were injected with control MO or hipk2 spl MO with or without 100 pg (top and second panels) or 200 pg (bottom panels) of MO-insensitive mouse Hipk2 KN mRNA, as indicated. Panels show whole mount in situ hybridization of OTM:d2EGFP (d2EGFP) in OTM:d2EGFP-transgenic zebrafish embryos (top panels) or tbx6 or ntla and dlx3b in non-transgenic zebrafish embryos (second and bottom panels) fixed at the indicated stages.
(K) PP1c functions upstream of GSK-3β. Embryos were injected with control MO or ppp1caa MO as indicated, and then treated with DMSO or 10 μM of the GSK-3β inhibitor BIO from 5 to 8 hpf. Panels show whole mount in situ hybridization of tbx6 in embryos.
(L) PP1c and Hipk2 cooperate to regulate CE. Embryos were injected with control MO or a low dose of hipk2 MO (1 ng) or ppp1caa MO (2 ng) as indicated. Panels show whole mount in situ hybridization of ntla and dlx3b in embryos fixed at 10 hpf. In (I–L), the percentages of embryos showing similar gene expression patterns and total number of MO-injected embryos (n) are shown in each image. Scale bar = 200 μm.
Image
Figure Caption
Acknowledgments
This image is the copyrighted work of the attributed author or publisher, and
ZFIN has permission only to display this image to its users.
Additional permissions should be obtained from the applicable author or publisher of the image.
Full text @ Cell Rep.