- Title
-
Inositol hexakisphosphate kinase-2 acts as an effector of the vertebrate Hedgehog pathway
- Authors
- Sarmah, B., and Wente, S.R.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
IP6K2 activity is required for normal development of craniofacial and somite structures. (A–C) Lateral view of uninjected (A), ip6k2SPMO-injected (B), and ip6k2SPMO + ip6k2 mRNA-coinjected (C) live embryos at 24 hpf. Eighty percent of the ip6k2SPMO embryos had a small head and reduced trunk; both the defects were rescued by ip6k2 mRNA coinjection. Effective ip6k2SPMO suppression of splicing was confirmed by RT-PCR (Fig. S3 A and B). (D–K) Ventral view of Alcian blue stained head skeleton of 5-d-old uninjected control (D), ip6k2SPMO (E–G), ip6k2SPMO + ip6k2 mRNA (H), ipk2SPMO (I), and ipk1MO1 (J and K)-injected embryos. The anterior neurocranium was mostly maintained in 40% of ip6k2MO embryos, but distorted or reduced in 60% of embryos (F and G). The pharyngeal skeleton was affected in 92% embryos injected (n = 90). ip6k2 mRNA coinjection restored normal craniofacial skeleton (H). Craniofacial skeleton was altered in ipk2SPMO embryos (I). No craniofacial deformity was evident in ipk1MO1 embryos (J and K). e, eye; m, Meckel′s cartilage; pq, palatoquadrate; ch, ceratohyal; cb 3–7, ceratobranchial arches 3–7; eth, ethmoid plate. |
|
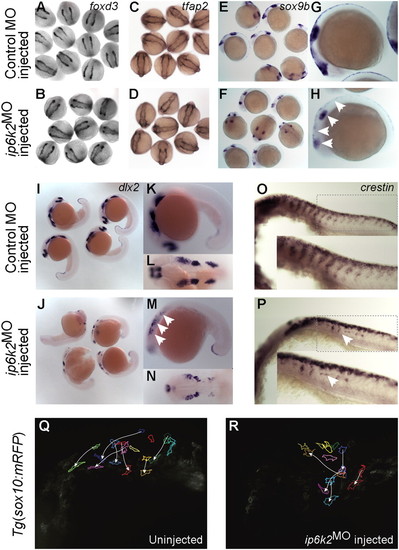
IP6K2 is critical for NCC development and migration. (A–P) Whole-mount in situ hybridizations for NC marker gene expression specifying distinct stages of NC development: (A–D) specification (foxd3 and tfap2 at 11 hpf), (E and H) maintenance (sox9b at 14 hpf), and (I–P) migration (dlx2 and crestin, 22 hpf) in control MO (A, C, E, G, I, K, L, and O) and ip6k2ATGMO (B, D, F, H, J, M, N, and P)-injected embryos. Arrowheads indicate distinct differences in the expression of sox9b (E–H), dlx2 (I–N), and crestin (O and P) between control MO and ip6k2ATGMO embryos. (Q and R) Analysis of NCC migration in real time using Tg(sox10(7.2):mrfp) fish (17) (see SI Materials and Methods for details). Fluorescence images (frames) for the hindbrain and eye regions were captured from a 6-h time-lapse sequence, with dorsal toward the top and anterior to the left. The 0-min time point represents 16-hpf stage. Images of uninjected (Q) and ip6k2ATGMO-injected (R) embryos show an overlay of first and last images onto which the tracings of migratory paths of CNCC were superimposed. Arrowheads indicate end point of each cell′s trajectory. EXPRESSION / LABELING:
PHENOTYPE:
|
|
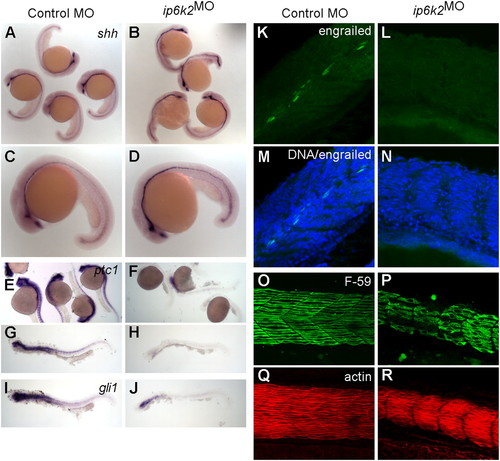
IP6K2 is required for Hh target gene expression during development. (A–J) Whole-mount in situ hybridizations showing expression of Hh target genes in zebrafish embryos (24-hpf stage) injected with either control MO (column 1) or ip6k2SPMO (column 2). Expression of shh (A–D) was slightly elevated, whereas ptc1 (E–H) and gli1 (I and J) expression was down-regulated in ip6k2SPMO (B, D, F, H, and J) compared with controls (A, C, E, G, and I). (K–N) Whole-mount immunohistochemistry showing engrailed protein levels (detected by 4D9 antibody) in control MO (K) or ip6k2SPMO (L) embryos at 24 hpf. Engrailed was not detected in ip6k2SPMO embryos (L). M and N overlay engrailed immunohistochemistry and TO-PRO3-stained DNA. (O–R) Lateral views of embryos at 24 hpf stained for myosin heavy chain using F59 antibody to visualize slow muscle cells (O and P) and F-actin using rhodamine-phalloidin to reveal both fast and slow muscle cells (Q and R). Dorsal is toward the top and anterior to the left. |
|
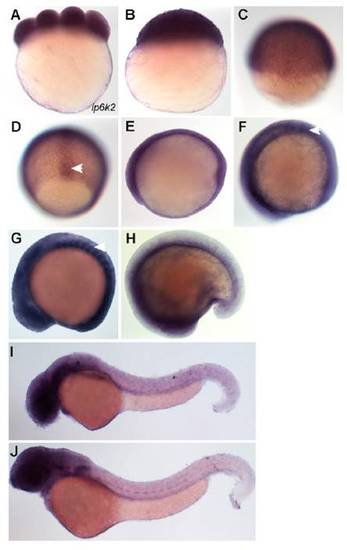
ip6k2 is expressed during zebrafish embryogenesis. Gene expression was analyzed during early embryogenesis. (A) Eight-cell-stage embryo showing maternally expressed ip6k2 mRNA. (B and C) Lateral views of blastula stage (4 hpf) embryo (B) and embryo at early gastrulation stage (6 hpf) (C) showing ubiquitous ip6k2 expression. (D) Dorsal view showing ip6k2 expression in axial cells (arrowhead) at midgastrulation (8 hpf). (E) Lateral view showing ubiquitous ip6k2 expression at 10 hpf. (F–H) Lateral views showing ip6k2 expression during segmentation stages. At 11 hpf, ip6k2 expression is detected in the neural plate and somites (arrowhead) (F). ip6k2 expression is enriched in somites (arrowhead), both forebrain and hindbrain regions, and developing eyes at 15 hpf (G); expression is reduced at 18 hpf (H). (I and J) Lateral views showing ip6k2 expression in retina, otic vesicle, and pronephric duct at 40 hpf (I) and in central nervous system at 60 hpf (J). |
|
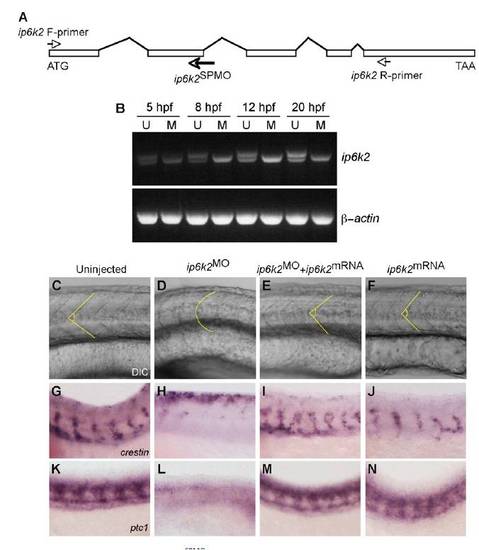
ip6k2 mRNA rescues the Hh-related phenotypes in ip6k2SPMO-injected embryos. (A) Schematic of ip6k2 genomic organization with exons (rectangular boxes) and introns (lines). Arrows indicate relative positions of ip6k2SPMO and oligonucleotide primers used to amplify ip6k2 cDNA. (B) ip6k2SPMO blocks splicing at exon2-intron2 junction. Total RNA prepared from uninjected (U) and ip6k2SPMO injected (M) embryos at different stage (5–20 hpf) were analyzed by twostep RT-PCR using ip6k2-F and -R primers (shown above). Reduced sized, aberrantly spliced ip6k2 product was detectable at 5 hpf and later time points in ip6k2SPMO-injected embryos (Upper). PCRs with β-actin-specific primers served as controls (Lower). (C–N) ip6k2 mRNA injection reverses inhibitory effects of ip6k2SPMO on somite development (C–F), NCC migration (G–J), and Hh target gene expression (K–N). Anterior is toward the left and dorsal toward the top. (C) Lateral view of chevron-shaped somites of a live uninjected embryo at 24 hpf. (D and E) Rescue of U-shaped somites in embryos injected with ip6k2SPMO (D) by coinjecting ip6k2 mRNA (E). (F) ip6k2 mRNA injection alone did not have any effect. Dotted lines indicate somite boundaries. (G–J) Whole- mount in situ hybridizations showing crestin expression in trunk NCC in uninjected (G), ip6k2SPMO-injected (H), ip6k2SPMO + ip6k2 mRNA-coinjected (I), and ip6k2 mRNA injected (J) embryos at 24 hpf. Expression of crestin in ip6k2SPMO-injected embryos was restored by ip6k2 mRNA coinjection (H and I). ip6k2 mRNA injection alone did not alter crestin expression (J). (K–N) Whole-mount in situ hybridizations showing ptc1 expression in uninjected (K), ip6k2SPMO-injected (L), ip6k2SPMO + ip6k2 mRNA-coinjected (M), and ip6k2 mRNA-injected (N) embryos at 24 hpf. It is apparent that ip6k2 mRNA coinjection rescued ptc1 expression in embryos injected with ip6k2SPMO (L and M), whereas ip6k2 mRNA injection alone had no effect (N). |
|
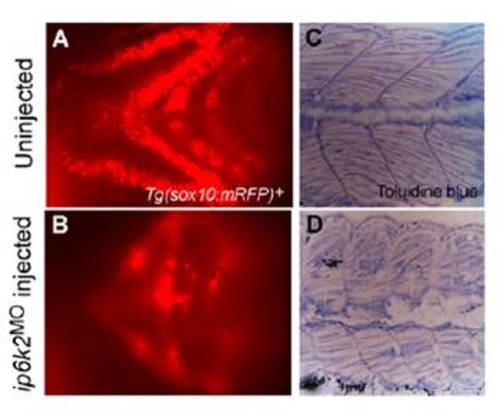
ip6k2 knockdown perturbs craniofacial development and somite structures. (A and B) Ventral view of the head region showing cartilage fluorescence of 3-d-old Tg(sox10(7.2):mrfp) embryo (A) and embryo injected with ip6k2ATGMO (B). Reduced mRFP expression in the ip6k2ATGMO embryos reflects a lack of pharyngeal arches. (C and D) Toluidine blue staining of the trunk region of 5-d-old uninjected (C) and ip6k2ATGMO-injected (D) embryo. The embryos were fixed and embedded in plastic resin, sectioned, and stained with toluidine blue. Sagittal sections through the midtrunk region were shown. The somites in the ip6k2ATGMO embryo were defective in shape and muscle fiber organization. |