- Title
-
Glial cell line-derived neurotrophic factor defines the path of developing and regenerating axons in the lateral line system of zebrafish
- Authors
- Schuster, K., Dambly-Chaudière, C., and Ghysen, A.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
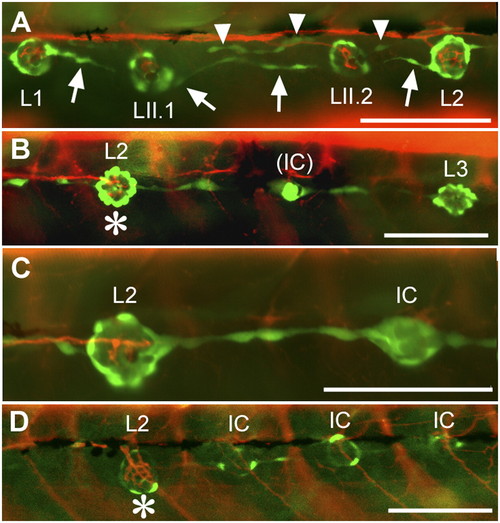
Innervation defects in Huc:kaede, Et20:gfp early larvae. (A) Normal pattern in a control larva at 4 dpf. Nerve branches extend to and arborize in each neuromast. Interneuromast cells deposited by the primordium between L1 and L2 (arrows) have moved ventrally ahead of the primII-derived neuromasts, LII.1 and LII.2. Interneuromast cells deposited by primII can also be observed close to the myoseptum (arrowheads). (B) Peripheral afferent axons stop at L2 (asterisk) in a 3-dpf gdnf, ret1-MO1 fish. An intercalary neuromast (IC) is beginning to develop between L2 and L3. The red fibers posterior to L2 belong to motor axons innervating the somitic muscles. (C) A higher magnification photograph to show that no nerve branch extends beyond L2, in a 4-dpf double morphant fish. (D) Nerve stop at L2 (asterisk) in a 4-dpf gdnf-MO2 fish. Intercalary neuromasts have formed on all somitic borders posterior to the nerve arrest. Anterior is Left in all panels. (Scale bar, 100 mm.) |
|
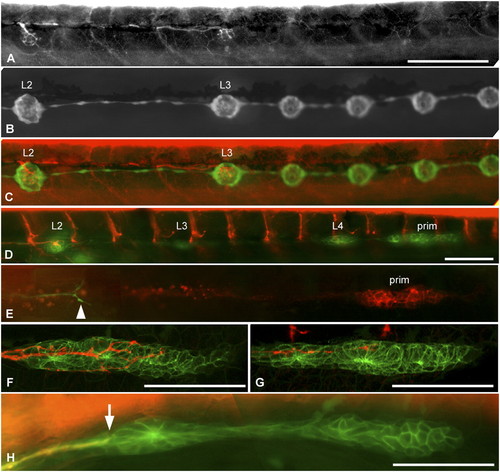
Innervation defects are due to precocious nerve arrest. (A–C) Nerve interruption at L3, in 4-dpf gdnf, ret1-MO1 double morphant fish. Nerve arrest (A) is associated with the formation of precocious intercalary neuromasts distal to the interruption (B and C). (D and E) Nerve interruption in double morphant embryos is manifest at a time when the primordium is still migrating in photoconverted Huc:kaede, cldnb:gfp (D) or in Huc:kaede, cxcr4b:rfp (E, arrowhead marks the nerve ending) embryos. (F and G) Arborization of sensory neurites accompanying the migrating primordium at 35 hpf in a control (F) and a gdnf, ret1-MO1 double morphant embryo (G). (H) Nerve lagging behind the primordium (arrow) in an nbt:dsred, cldnb:gfp embryo injected with 0.15 mM ret1-MO2. L1 is about to be deposited. E and H are composite pictures assembled from two consecutive planes in Z-stacks. Anterior is left in all panels, and the primordium is migrating to the right in D–H. (Scale bar, 100 mm.) |
|
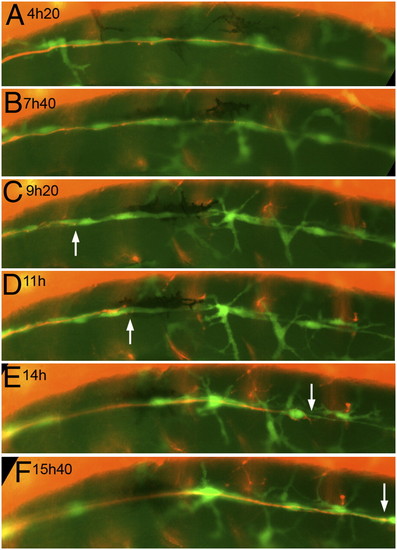
Still panels from Movie S3. The nerve was cut just posterior to the ganglion at time 0 in a 3-dpf, nbt:dsred, foxd3:gfp embryo. (A–C) Progressive axonal degeneration; (C–F) progress of the regenerating growth cones (white arrows). (Scale bar, 100 mm.) EXPRESSION / LABELING:
|
|
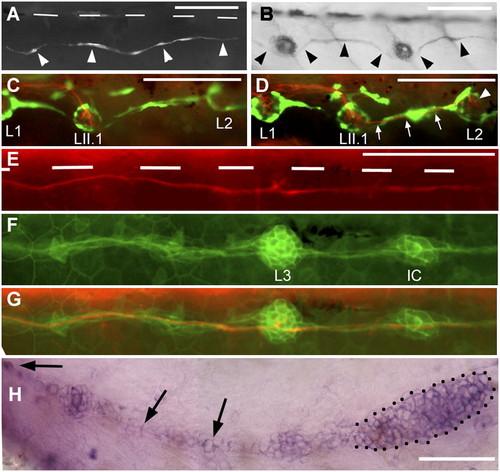
Regenerating axons can be guided by interneuromast cells. (A) After ablation of glial cells in the foxd3:gfp line, new glial cells extend posteriorly (arrowheads). (B) Their path resembles that of interneuromast cells (arrowheads) as visualized by alkaline phosphatase labeling. (C and D) After glial ablation between L1 and L2 in 3-dpf Huc:kaede, Et20:gfp fish, axons regrow along the myoseptum up to LII.1 (C) and follow the interneuromast cells (arrows) up to L2 (arrowhead in D). (E) Regenerating axons in a 54-hpf Huc:kaede, cldnb:gfp fish where the nerve had been cut at 30 hpf. White bars indicate the position of the horizontal myoseptum. (F) Trail of interneuromast cells in the same embryo; (G) merge. (H) Expression of gdnf in the primordium (dotted outline) and in the interneuromast cells (arrows) as revealed by in situ hybridization. (Scale bar, 100 mm.) A and H were assembled from two consecutive levels in Z-stacks. EXPRESSION / LABELING:
|
|
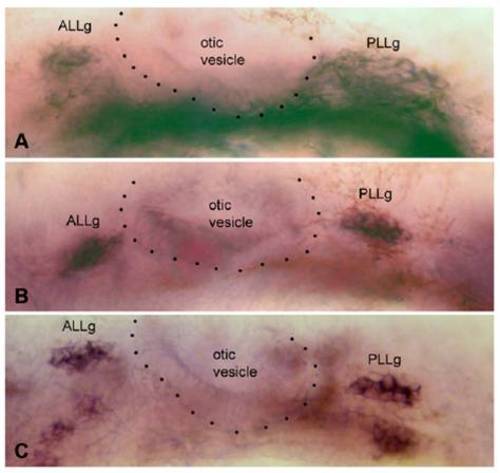
Expression of gfr1a, gfr1b, and ret1 in the anterior (ALLg) and posterior (PLLg) lateral line ganglia, on either side of the otic vesicle (dotted outline), in 35-hpf embryos. C has been assembled from two slightly different focal planes. EXPRESSION / LABELING:
|