- Title
-
Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries
- Authors
- Hogan, B.M., Herpers, R., Witte, M., Heloterä, H., Alitalo, K., Duckers, H.J., and Schulte-Merker, S.
- Source
- Full text @ Development
|
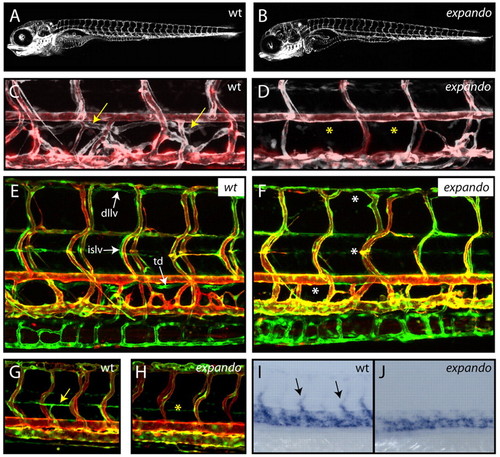
Zebrafish expando mutants lack lymphatic vessels and venous sprouting. (A,B) Lateral views of TG(fli1:GFP) expression in wild-type (wt; A) and expando mutant (B) larvae at 5 dpf demonstrate that overall vascular patterning is not perturbed in expando mutants. (C,D) Overlay image of angiogram (red) and TG(fli1:GFP) (white) indicating the presence of blood flow in wild-type (C) and mutant (D) embryos. The thoracic duct (arrows) is present in the wild type, but absent (asterisks) from mutant larvae. (E,F) Lateral views of wild type (E) and expando mutant (F) in a double-transgenic TG(fli1:GFP), TG(kdr-l:Cherry) background. All lymphatic vessels are absent in mutants at 5 dpf. Arrows indicate the thoracic duct (td), intersegmental lymphatic vessels (islv) and dorsal longitudinal lymphatic vessels (dllv) in wild-type embryos. Asterisks indicate their absence in expando mutants. (G,H) Parachordal lymphangioblasts (PLs) are absent from the horizontal myoseptum in expando mutants (asterisk in H) as compared with wild-type siblings (arrow in G indicates PL) in the double-transgenic TG(fli1:GFP), TG(kdr-l:Cherry) background at 54 hpf. (I,J) tie2 expression in venous sprouts (arrows in I) is absent in expando mutants (J). n=6/29 embryos scored and confirmed by genotyping. |
|
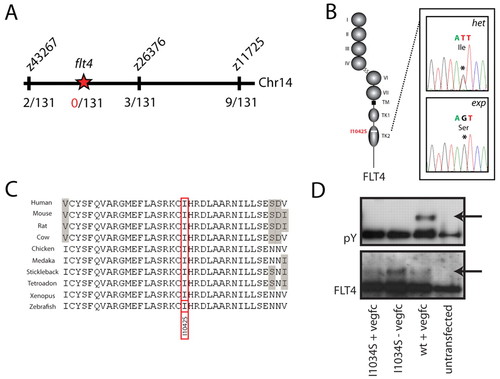
A mutation in the flt4 kinase domain is responsible for the expando phenotype. (A) Meiotic mapping links the expando locus to a region of chromosome 14 containing flt4. Analysis of 131 zebrafish embryos (262 meioses) identifies two recombination events at the marker z43267 and three independent recombinants at the marker z26376, thereby localising the mutation to a region containing flt4. (B,C) Sequencing of the coding exons of flt4 reveals a T>G mutation (see chromatogram in B) that changes a highly conserved isoleucine to a serine at position 1042 of the predicted zebrafish Flt4 protein sequence. Flt4 receptor structure is depicted, with Ig domain (I-VII), transmembrane domain (TM) and split tyrosine kinase domain (TK1 and TK2). Alignment of conserved sequences (C) demonstrates that I1042 is a conserved residue, with grey shading indicating non-conserved residues. (D) Tyrosine phosphorylation (pY; upper panel, phosphotyrosine blot) was seen upon in vitro stimulation with VEGFC of wild-type human FLT4, but not of I1034S mutant FLT4. Lower panel (Flt4 protein blot) indicates the presence of FLT4 protein post-transfection in the assayed samples. |
|
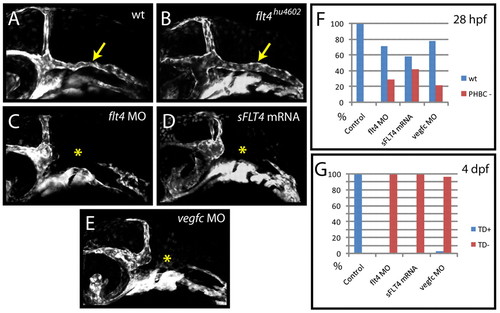
Zebrafish flt4hu4602 mutants lack the PHBC defects observed upon flt4 MO, vegfc MO or sFLT4 mRNA injection. (A-E) Angiogenesis of the primordial hindbrain channel (PHBC) fails to occur in flt4 MO-injected (5 ng/embryo) (C), sFLT4 mRNA-injected (200 pg/embryo) (D) and vegfc MO-injected (E) embryos (yellow asterisk) as compared with wild-type controls (A), but is unaffected (yellow arrow) in individually genotyped flt4hu4602 mutants (B) at 28 hpf. (F,G) Angiogenesis of the PHBC and lymphangiogenesis respond differently to injection of flt4 MO, vegfc MO and sFLT4 mRNA. Injection of flt4 MO (5 ng/embryo) inhibits PHBC development in 29% of embryos at 28 hpf but inhibits lymphangiogenesis [thoracic duct (TD) scored as readout] with 100% efficiency at 4 dpf in the same injection (n=55 embryos scored). vegfc MO (5 ng/embryo) inhibits PHBC development in 22% of embryos (n=41) and TD development in 97% of embryos in the same injection (n=37). sFLT4 mRNA (200 pg/embryo) inhibits PHBC development in 42% of embryos and TD development in 100% of embryos in the same injection (n=120). Uninjected control embryos showed normal PHBC and TD development in 100% of cases (n=20) at 28 hpf and 4 dpf (F,G). |
|
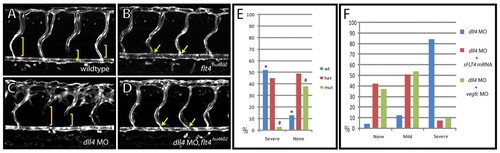
The dll4 morphant hyperbranching phenotype is rescued in flt4hu4602 mutants, vegfc morphants and sFLT4 mRNA-injected embryos. (A-D) Lateral views of uninjected control (A) and flt4hu4602 mutant (B) as compared with wild-type (C) and flt4hu4602 mutant (D) zebrafish embryos injected with dll4 MO. Images were taken in the TG(flt1:YFP)hu4624 background, labelling trunk arteries to reveal the failure to form intersegmental venous sprouts in expando mutants (B, yellow arrows). The dll4 morphant arterial hyperbranching phenotype (C) is rescued in flt4hu4602 mutants (D). Venous sprouts were identified by the absence of flt1:YFP expression in ISVs (brackets). Embryos at 72 hpf. (E) Arterial hyperbranching phenotypes were scored post-injection of dll4 MO and subsequently genotyped. The mutant genotype was almost completely absent from the severe category (e.g. C) (1/29 embryos, *P<0.01 by χ2 test) and was enriched in the category not displaying hyperbranching (e.g. D) (21/55 embryos, #P<0.01 by χ2 test). (F) In transgenic TG(flt1:YFP)hu4624 embryos, co-injection with dll4 MO together with vegfc MO (green bars, n=57 embryos), or of dll4 MO together with sFLT4 mRNA (200 pg/embryo) (red bars, n=67 embryos), led to rescue of the arterial hyperbranching phenotype observed upon injection of dll4 MO alone (blue bars, n=81 embryos). Arterial hyperbranching phenotypes were categorised as no hyperbranching (none), mild or severe (see Fig. S1 in the supplementary material). EXPRESSION / LABELING:
PHENOTYPE:
|
|
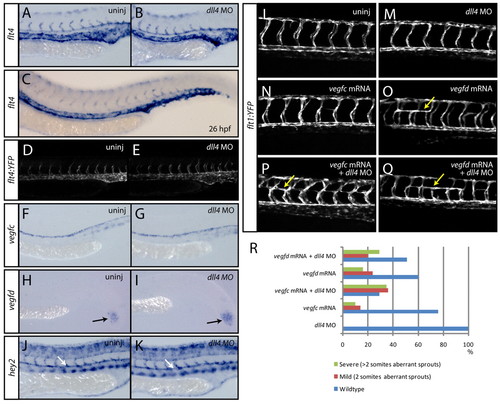
Sprouting intersegmental arteries express flt4 and are sensitised to exogenously introduced Vegfc in the absence of dll4. (A-E) At 24 hpf, the expression of flt4 is present in all arterial cells (A) and is unaltered upon loss of dll4 (B) (n=40 uninjected control and n=40 dll4 MO-injected embryos scored). By 26 hpf (C), flt4 is downregulated in the dorsal aorta (n=20 embryos). Analysis of a flt4 promoter-driven transgenic line identifies YFP expression in all arterial cells at 24 hpf (D). The expression of YFP is unaltered in dll4 MO-injected embryos (E). (F,G) At 24 hpf, the expression of vegfc is present in the dorsal aorta (F) and is unaltered in dll4 MO-injected embryos (G) (n=20 uninjected and n=20 MO-injected embryos). (H,I) The expression of vegfd in the tailbud is unaltered in dll4 MO-injected (I) as compared with uninjected control (H) embryos (n=20 uninjected and n=20 MO-injected embryos). (J,K) The expression of the arterial marker gene hey2 is unaltered in dll4 MO-injected (K) as compared with uninjected control (J) embryos (n=20 uninjected and n=20 MO-injected embryos). (L-R) dll4 morphants are sensitised to exogenously introduced vegfc mRNA during arterial angiogenesis. Forced expression by the injection of mRNA encoding full-length zebrafish Vegfc into dll4 MO-injected embryos (n=133) (P) leads to aberrant ectopic bilateral turning of ISAs by 28 hpf (yellow arrows), which was never observed in wild-type embryos (L) or in those injected with dll4 MO only (n=90) (M). ISAs rarely turn bilaterally upon injection of vegfc mRNA only (n=106) (N), whereas injection of full-length vegfd mRNA robustly showed this phenotype without (n=211) (O) or with (n=227) (Q) dll4 MO. For each of the conditions tested, the percentage of embryos showing wild-type, mild (aberrant ISAs spanning two somites) or severe (aberrant ISAs spanning more than two somites) ISA phenotypes is shown in R. |
|
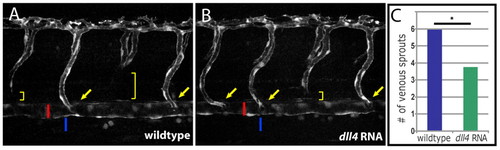
Overexpression of dll4 reduces sprouting from the PCV. (A,B) Injection of mRNA encoding full-length Dll4 into TG(flt1:YFP)hu4624 zebrafish embryos leads to a reduction in the number of venous sprouts in injected (B) as compared with uninjected control (A) embryos at 52 hpf. ISAs that connect directly to the dorsal aorta (red bar) are indicated by yellow arrows. Venous spouts (yellow brackets) can be readily identified by the absence of TG(flt1:YFP)hu4624 expression in the ventral component of an intersegmental vessel. Venous sprouts connect directly to the posterior cardinal vein (blue bar) from which they derive during secondary sprouting. (C) Quantification of the number of venous sprouts (y-axis) on one side of the embryo across 12 segments anterior to the cloaca (n=23 embryos scored for both dll4 MO-injected and uninjected controls). dll4 MO-injected embryos show a significant decrease in the number of intersegmental venous sprouts (P<0.001, Mann-Whitney rank sum test). EXPRESSION / LABELING:
|
|
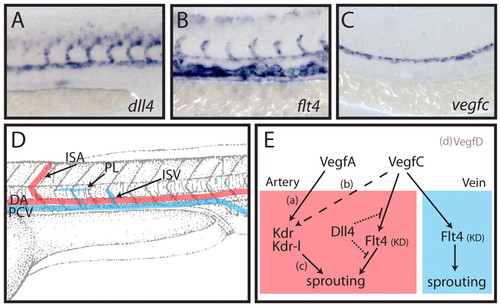
Dll4 inhibits arterial Vegfc/Flt4 signalling in the developing zebrafish trunk. (A-C) Expression analysis at 24 hpf for dll4 (A), flt4 (B) and vegfc (C) indicates the arterial restricted expression of dll4, expression of flt4 in all venous and arterial cells, and expression of vegfc restricted to the dorsal aorta. (D) Schematic overview of the developing vasculature in the zebrafish trunk. DA, dorsal aorta; PCV, posterior cardinal vein; ISA, intersegmental artery; ISV, intersegmental vein; PL, parachordal lymphangioblast. (E) Working model of the interplay between Dll4 and Vegfc/Flt4 signalling. Previous studies have shown that vegfa (vegfaa and vegfab duplicates in zebrafish) is necessary for ISA development [a (Bahary et al., 2007; Nasevicius et al., 2000)], that vegfc influences (dashed line) ISA development [b (Covassin et al., 2006)] and that the receptors kdr and kdr-l mediate intracellular signalling to control ISA development [c (Bahary et al., 2007; Covassin et al., 2009; Covassin et al., 2006; Habeck et al., 2002; Lawson et al., 2003; Meng et al., 2008)]. We have previously shown that vegfd is unlikely to play a role in ISA development owing to its restricted expression [d (Hogan et al., 2009)]. Here we demonstrate that signalling through the Flt4 kinase domain (KD) does not contribute to ISA development as it is suppressed by Dll4 (dotted lines indicate that the mechanism of suppression is unknown). In the absence of Dll4, endogenous Vegfc drives a Flt4 (KD)-dependent ISA hyperbranching phenotype. In the vein (which does not express dll4), Vegfc/Flt4 signalling is indispensable for normal venous angiogenesis and lymphangiogenesis. EXPRESSION / LABELING:
|
|
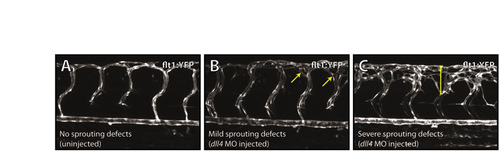
Categories of hyperbranching as scored in Fig. 4A-C. Examples of embryos categorised as having no sprouting defects (wild type) (A), mild sprouting defects (B), or severe sprouting defects (C) at 72 hpf, as taken from the experiment summarised in Fig. 4F. Arrows in B and bracket in C indicate regions of hyperbranching. |
|
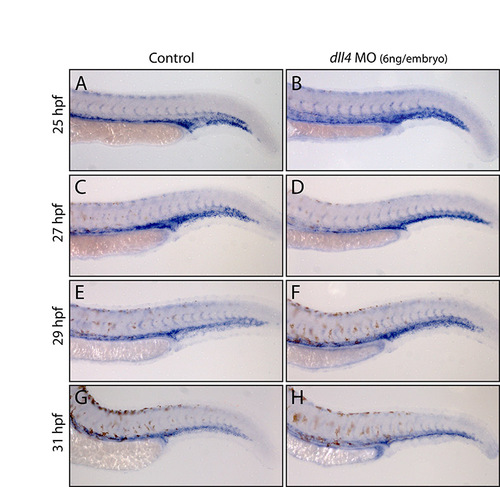
No appreciable change in arterial flt4 expression in dll4 morphants between 25 and 31 hpf. (A-H) Expression of flt4 in trunk arteries and veins in stage-matched wild-type uninjected control and dll4 MO-injected (6 ng/embryo) embryos. No difference was observed at 25 hpf (A,B) between control (n=15) and morphant (n=28) embryos. At 27 hpf (C,D), no difference was seen between carefully stage-matched control (n=18) and morphant (n=29) embryos. Some morphants that were clearly developmentally delayed (n=11) showed higher levels of flt4 expression than controls, consistent with the dynamic downregulation of ISA flt4 expression between 25 and 29 hpf, and demonstrating the need to stage match rather than age match embryos during this analysis. At 29 hpf (E,F; n=16 control and n=34 morphant embryos) and at 31 hpf (G,H; n=16 control and n=33 morphant embryos), no difference was observed in the ISA expression of flt4 between control and morphant embryos. Embryos from the same MO-injection sessions were grown to 3 dpf and the robust dll4 MO-induced hyperbranching phenotype observed, demonstrating that the embryos analysed were morphants. |