- Title
-
Otopetrin 1 is required for otolith formation in the zebrafish Danio rerio
- Authors
- Hughes, I., Blasiole, B., Huss, D., Warchol, M.E., Rath, N.P., Hurle, B., Ignatova, E., David Dickman, J., Thalmann, R., Levenson, R., and Ornitz, D.M.
- Source
- Full text @ Dev. Biol.
|
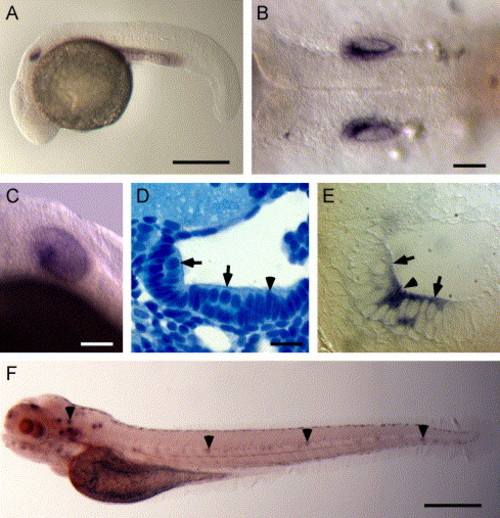
Whole mount in situ hybridization analysis of otop1 mRNA expression. (A) Lateral view of a 24 hpf embryo showing significant expression of otop1 in the ventral half of the developing otic vesicle. (B) Dorsal view at 24 hpf showing otop1 expression. (C) Lateral view of 3 dpf larva showing otop1 mRNA expression in the sensory epithelium. (D) Four-μm plastic section of the otocyst of a 3 dpf fish (dorsal is up, lateral to the right) stained with Richardson′s stain (100×). Pale apical cells with round nuclei (arrow) are hair cells. Cells with elongated, densely staining nuclei (arrowheads) are precursor cells within the macular growth zone. (E) Similar 4 μm plastic section of a 3 dpf fish showing otop1 mRNA localized to the luminal cells of the otocyst and adjacent cells in the monolayer, consistent with expression in the mature and developing hair cells (100x). (F) Lateral view of a 5 dpf larva showing otop1 expression along the entire length of the animal in the anterior and lateral line organs (arrowheads). Scale bars: A, 50 μm; B–C, 250 μm; D–E, 10 μm; and F, 50 μm. |
|
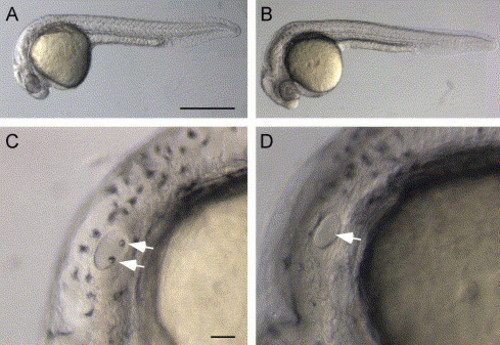
Absence of otolith formation in otop1 morphant fish. (A) Lateral view of an uninjected wild-type control at 30 hpf. (B) Lateral view of a 30 hpf morphant fish injected with 10 ng MO-1. Otop1 morphant fish are morphologically normal at all stages of development but lack otolith formation. (C and D) Lateral view of control and morphant 30 hpf otocysts. Otoliths are located at the poles of the developing otocyst of a control fish (arrows) (C) but are absent in the 10-ng MO-1-injected animals (arrow) (D). Scale bars: A–B, 50 μm; and C–D, 250 μm. |
|
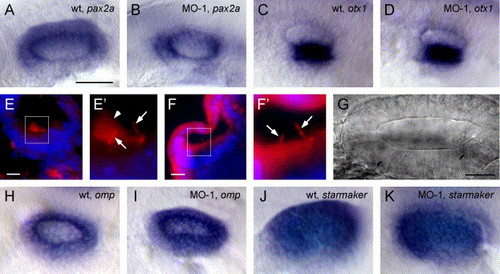
Normal gene expression and otocyst morphogenesis in morphants. (A) The pax2a expression in wild-type and (B) morphant otocyst. (C) otx1 expression in a wild-type and (D) morphant otocyst. (E) Immunohistochemistry for acetylated tubulin on 10 μm frozen sections through a 28 hpf wild-type otocyst. Tether cell kinocilia (arrow) and otolith (arrowhead) are evident in magnified insert (E′). (F) Morphant otocyst examined for acetylated tubulin. Tether cell kinocilia are evident in magnified insert (F′) (arrow) but no otolith seeding particles were present. (G) DIC image of the otocyst of a morphant fish (100×) at 24 hpf. No aggregated matrix was visible in several ears examined in this manner. (H) omp expression in wild-type and (I) morphant otocyst. (J) starmaker expression in wild-type and (K) morphant otocyst. A–D and H–K are whole mount in situ hybridizations of 24 hpf embryos. All otop1 morphant fish were injected with 4 ng MO-1 at the one-cell stage. Rostral is on the left. Scale bars: A–D, H–K, 250 μm; E–F, 10 μm; G, 25 μm. EXPRESSION / LABELING:
|
|
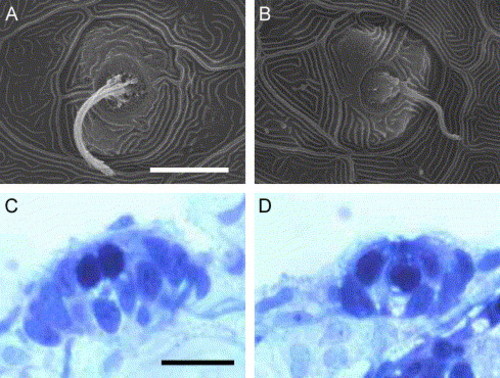
Normal formation of the lateral line system in otop1 morphant fish. (A–B) SEM image of a 4 dpf WT (A) and morphant (B) posterior lateral line neuromast. The cupula and kinocilia bundle has formed normally in the morphant animal. (C and D) Four-μm plastic section through a 7 dpf uninjected control (C) and 10 ng MO-1-injected morphant anterior neuromast showing a similar complement of hair cells (pale, apical cells with round nuclei) and supporting cells. Scale bars indicate 10 μm. C and D are stained with Richardson′s stain. |
|
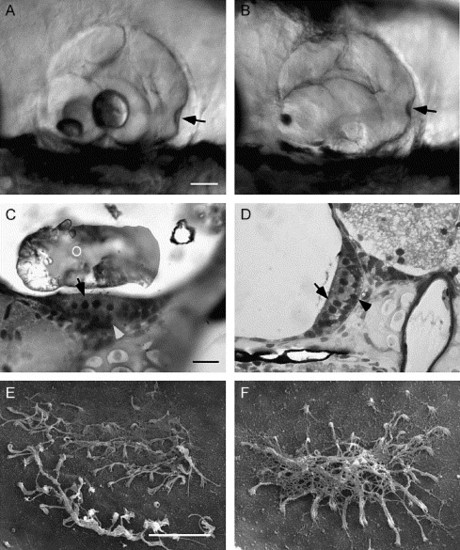
Morphogenesis of the otocyst and sensory epithelium. (A and B) Lateral view of a 5 dpf wild-type (A) and morphant (B) otocyst. In the wild-type fish, the utricular otolith is smaller and located in the rostral portion of the otocyst, the saccular otolith is larger and located centrally. The semicircular canals and ampullae (arrowhead) are similar in control and in 10 ng MO-1 morphant fish with otolith agenesis. (C) Plastic section through the utricular macula of a 7 dpf wild-type fish (100×) showing development of the hair cells (pale apical cells, arrow) and supporting cells (darker basal cells, arrowhead). (D) Plastic section of a 7 dpf morphant saccular macula showing a similar distribution of cell types. (E and F) Scanning electron micrographs of a wild-type (E) and morphant (F) utricular otolithic membrane at 7 dpf. Removal of the otolith disrupted the wild-type otolithic membrane. Morphant fish formed an otolithic membrane in the absence of the otolith. The otolithic membrane is fibrous and connects the stereocilia of all hair cells in the macula. O, otolith. Scale bars: A–B, 250 μm; C–D, 10 μm; E–F, 10 μm. |
|
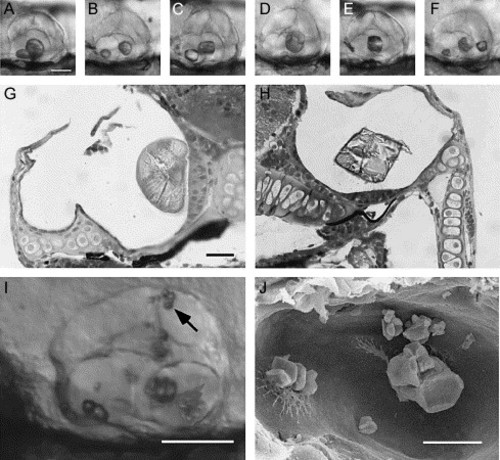
Delayed otolith formation and dysmorphology in otop1 morphant fish. (A–F) Lateral views of 7 dpf otocysts (rostral to left) of a wild-type (A) and 1 ng MO-1-injected fish (B–F). (A) Wild-type fish develop two ovoid otoliths. (B) Delayed otoliths in morphant fish can appear similar to wild type in shape and location. (C) Otoliths that are identical in location, but with an oblong shape and distinct straight edges. (D) Formation of a single otolith that is larger than a wild-type otolith and attached to the saccular macula. The irregular shape and large size may indicate fusion of the early otolith seeding particles. (E) A single cuboidal otolith located in the saccular sensory macula. (F) Multiple otoliths with polyhedral structures. The posterior-most otolith did not appear to be attached to a sensory macula. (G) Four-μm plastic section through a 7 dpf 1 ng MO-1-injected morphant otocyst showing a wild-type-like otolith attached to the saccular macula. Richardson′s stain identifies concentric rings of organic matrix within the otolith structure. (H) Four-μm plastic section through the opposite otocyst showing an angular otolith on the utricular sensory patch with no obvious organic matrix within the crystal. (I) Lateral view of a 4 dpf otocyst (rostral to left) from an 8-ng MO-1-injected fish. At 4 dpf, numerous irregularly shaped otoliths are found throughout the otic cavity and in the semicircular canal (arrow). (J) SEM image of the same 4 dpf morphant otocyst. Note the presence of aggregates of crystals attached to the utricular and saccular otolithic membranes and additional free-floating crystals on the otocyst wall (several otolith particles were lost in preparation). Scale bar for A–F is 250 μm; G–H is 10 μm; I is 250 μm; and J is 25 μm. |
|
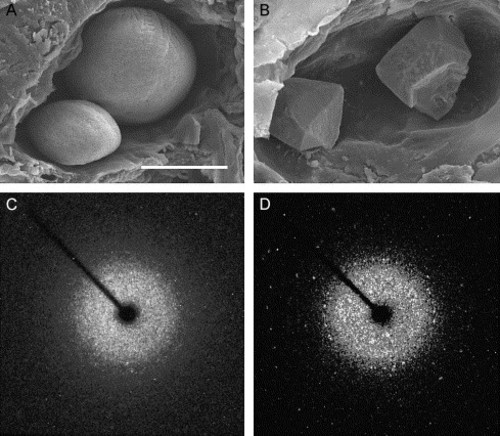
Calcitic otolith formation in otop1 morphant fish. (A) Scanning electron microscopy of the otic cavity of a 7 dpf uninjected age-matched control showing a smaller utricular (left) and larger saccular otolith. Both otoliths are rounded and cover the entire sensory maculae. (B) Eight-ng MO-1-injected morphant otic cavity at 7 dpf. Otoliths are angular. The sensory epithelium is visible below the utricular otolith. The morphant otoliths resembled inorganic crystals instead of organic calcification. (C) The 360° rotation image of a single crystal X-ray diffraction of a wild-type 7 dpf otolith. Little prominent diffraction pattern is present, indicating that calcium carbonate aragonite crystallites are arranged in a dustlike mosaic pattern across the surface of the otolith. (D) Single crystal X-ray diffraction of a morphant otolith showing that otoliths similar to those above (B) behave as a single crystal. The unit cell derived from this diffraction pattern was consistent with the calcitic polymorph of calcium carbonate. Scale bar indicates 50 μm. |
Reprinted from Developmental Biology, 276(2), Hughes, I., Blasiole, B., Huss, D., Warchol, M.E., Rath, N.P., Hurle, B., Ignatova, E., David Dickman, J., Thalmann, R., Levenson, R., and Ornitz, D.M., Otopetrin 1 is required for otolith formation in the zebrafish Danio rerio, 391-402, Copyright (2004) with permission from Elsevier. Full text @ Dev. Biol.