- Title
-
A cluster of noninvoluting endocytic cells at the margin of the zebrafish blastoderm marks the site of embryonic shield formation
- Authors
- Cooper, M.S. and D'Amico, L.A.
- Source
- Full text @ Dev. Biol.
|
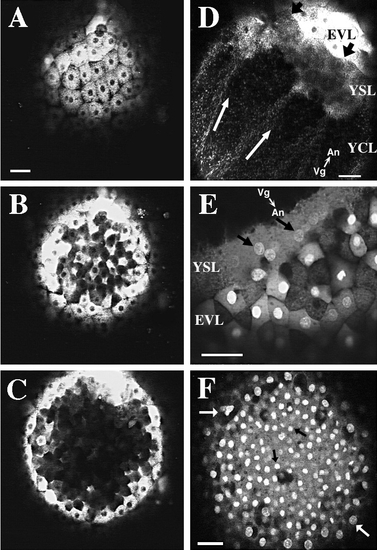
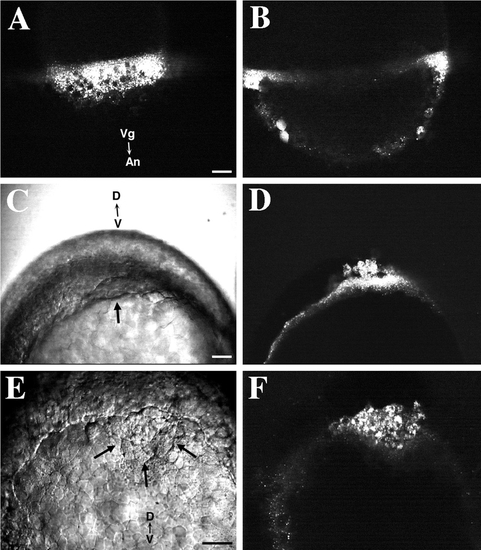
SYTO-11 labeling of mid-blastula-stage zebrafish embryos. Scale bars, 50 μm. Embryos at 1 k-cell stage to high-stage embryos were labeled with SYTO-11 and observed at later developmental stages. All panels are confocal micrographs. (A) SYTO-11 labeling is seen in the enveloping layer cells of a sphere-stage zebrafish embryo. SYTO-11 has been internalized by endocytosis into numerous fluorescent endosomes. Few EVL nuclei exhibit SYTO-11 labeling in this particular embryo. Animal pole view. (B) 15 μm deeper into the same embryo. Deep cells, which have delaminated from the EVL, contain numerous fluorescent endosomes in their cytoplasm. (C) 23 μm deeper into the blastoderm than B. No deep cells containing SYTO-11 are seen in the center of the blastoderm. Those deep cells that exhibit SYTO-11 labeling are in close proximity to the EVL epithelium. (D) SYTO-11 is also internalized by endocytosis into the cytoplasm of the yolk syncytial layer (YSL) and yolk cytoplasmic layer (YCL). Endosomes stream along linear tracks (white arrows) toward nuclei in the external YSL in an oblong-stage embryo. Black arrows point to the EVL – YSL margin. The animal-vegetal (An-Vg) axis is shown. (E) Nuclei in the YSL exhibit SYTO-11 labeling (black arrows). In this 30%-epiboly-stage embryo, a large fraction of EVL cells exhibit nuclear staining by SYTO-11. Vesicular labeling is observed throughout the cytoplasm of EVL cells and the YSL. Numerous fluorescent endosomes accumulate at the EVL-YSL margin. (F) Fixation releases SYTO-11 that has been internalized by endocytosis. Embryos were first labeled as above and then fixed with 4% paraformaldehyde at dome stage. The vesicular labeling pattern within the cytoplasm of EVL and deep cells is no longer observed. Instead, distinct nuclear labeling is observed in both cell populations. At this plane of focus, EVL nuclei (white arrows) are seen at the periphery of the image, and deep cell nuclei (dark arrows) are seen in the center. Animal pole view. |
|
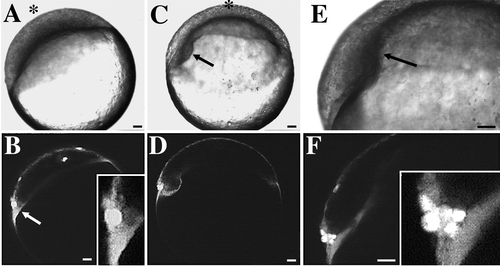
Noninvoluting endocytic marginal (NEM) cells in a late-blastula-stage embryo predict the site of embryonic shield formation. Scale bars, 50 μm. A–F show the development of an embryo previously labeled at dome stage with SYTO-11. Correlated Nomarski and confocal fluorescence micrographs at three different time points are shown. The animal pole is denoted by an asterisk. (A, B) 30%-epiboly; initial observation, 0:00 hr. (A) The blastoderm has begun to spread over the surface of the underlying yolk cell. (B) Bright cells (arrow) at the left margin of the blastoderm predict the location where the embryonic shield will form at 50%- to 60%-epiboly (see C and D). Inset (higher magnification) shows that these cells are in contact with the YSL. Several individual EVL cells that are scattered over the surface of the blastoderm are also labeled with SYTO-11. (C, D) 55%-epiboly; 1:30 hr. (C) The nascent embryonic shield is now visible as a thickening on the left side of the blastoderm (arrow). The embryo has rolled slightly into a new orientation. The animal pole now is at the top of the image. (D) The location of the fluorescently labeled NEM cell cluster in B predicted the site of embryonic shield formation. (E, F) 65%-epiboly; 2:10 hr. (E) Embryonic shield (arrow) extends toward animal pole. (F) During germ ring and embryonic shield formation, NEM cells remain at the dorsal margin of the blastoderm. NEM cells do not undergo involution during this time period. More cells are now visible in the NEM cell cluster. During embryonic shield formation, some deep cells in the NEM cell cluster move in the front of the dorsal blastoderm, at which point they become known as ‘‘forerunner’’ cells. Inset (higher magnification) shows that some forerunner cells are in contact with the YSL. |
|
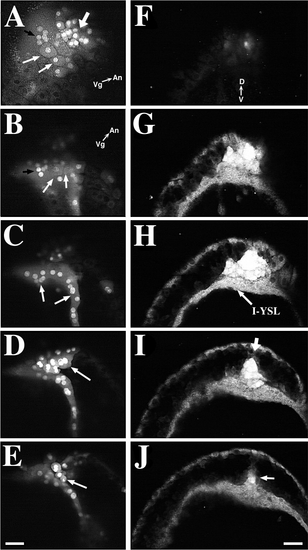
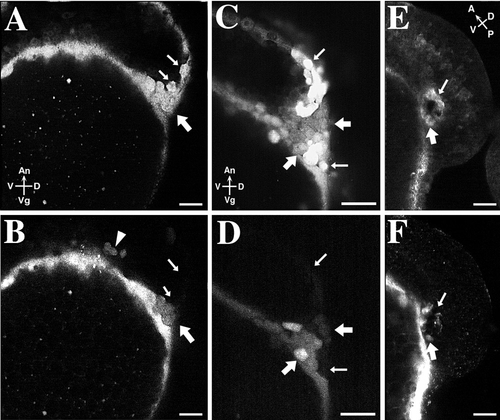
The forerunner cell cluster forms a wedge-shaped cap at the posterior limit of the embryonic axis during late-epiboly. Scale bars, 50 μm. (A–E) The forerunner cells form a wedge-shaped cap at the dorsal margin of the blastoderm. EVL cells, which derive from the original NEM cell cluster, overlie the forerunner cells. Forerunner cells are derived from deep NEM cells. The embryo is viewed from its dorsal side. The embryo was stained with SYTO-11 and red-emitting BODIPY 564/591 at 30%-epiboly and then imaged at 65%-epiboly. Scale bar, 50 μm. (A) SYTO-11-labeled cells include both EVL – NEM cells (thin white arrows) and deep (forerunner) cells (thick arrow). A group of brightly labeled nuclei (black arrow) in the YSL is located just vegetal to the NEM/forerunner cluster margin. This surface-view image was taken by using a dual excitation with 488- and 568-nm laser light, while fluorescence emission was collected >585 nm. Since SYTO-11 exhibits a red fluorescence, dual excitation of the specimen allows cell nuclei and endosomes, labeled with SYTO-11, and cellular cytoplasm, labeled with BODIPY 564/591, to be imaged simultaneously. The animal-vegetal (An-Vg) axis is shown. (B-E) A focus-through of the NEM/forerunner cell cluster in the same embryo as in F but rotated slightly counterclockwise and shifted into an oblique orientation. B-E were only collected with 488-nm excitation so that only SYTO-11 emission is seen. (B) This is a surface focal plane of the NEM/forerunner cell region showing the same EVL (white arrows) and YSL nuclei (black arrow) shown in A. (C) 15 μm deeper than B. Brightly labeled YSL nuclei (arrows are visible at this plane of focus. (D) 23 μm deeper into the embryo. Brightly labeled, multi-nucleate EVL-NEM cells (arrow) lie directly above the forerunner cell cluster. (E) 23 μm deeper into the embryo. SYTO-11-labeled deep cells (arrow) of the forerunner cell cluster are closely associated to overlying EVL-NEM cells. Panels F-J show a focus-through (15-μm steps) of a 80%-epiboly-stage embryo labeled with SYTO-11, starting from the posterior edge of the NEM/forerunner cell cluster and progressing toward the anterior end of the cell cluster. The embryo is viewed from the vegetal pole. The dorsal-ventral (D-V) axis is shown. (F) The focus-through starts at the level of the EVL-YSL boundary. (G) Labeled NEM/forerunner cells and YSL cytoplasm are prominent. (H) A distinct layer of forerunner cells lies directly above the internal YSL which contains numerous SYTO-11-filled endosomes. (I, J) Edges of the germ ring converge toward the embryonic midline which is defined by the dorsal apex (arrow) of the forerunner cell cluster. The NEM cell cluster in J also has an apex (arrow) pointing toward the anterior end of the embryo. The anterior apex of the forerunner cluster lies in a more ventral plane than the dorsal apex seen in H. |
|
The forerunner cell cluster does not involute during late epiboly. A-C and D-F show time-lapse recordings of two different embryos. The recordings are at slightly different focal planes and orientations. Scale bars, 50 μm. The embryo in D-F has a larger number of forerunner cells than the embryo in A-C. (A-C) A time-lapse series of an embryo stained with BODIPY 505/515. Vegetal pole view. The embryo in A-C is filmed with the blastopore facing down against the microscope coverslip. A wedge-shaped group of NEM/forerunner cells is visible at the leading edge of the blastoderm from 70%- to 90%-epiboly (black arrow). The yolk cell is brightly labeled with BODIPY 505/515. (A) The embryo is at 70%-epiboly. (B) At 90%-epiboly, the fore-runner cell cluster becomes depressed into a more ventral position as the germ ring shrinks in circumference. The forerunner cells separate from overlying EVL-NEM cells at this point in development. (C) The dorsal apex of the forerunner cell cluster is aligned with the presumptive tailbud, which is seen as a dorsal thickening (asterisk) in the germ ring. (D-F) The embryo of this time-lapse series was costained with BODIPY 505/515 (to label all the embryo’s blastomeres) and SYTO-11 (to enhance the visibility of the forerunner cluster). The time-lapse recording begins with an oblique view of the embryo’s dorsal side. This embryo was not immobilized in an agarose-ERM gel. During the recording, the embryo rolled into a position with its vegetal pole facing the coverslip (see E). The embryo was recentered in F. (D) Oblique view of the embryo at 70%-epiboly. The forerunner cell cluster, with more brightly labeled cells, is located at the leading margin of the blastoderm. (E) Vegetal pole view at 90%-epiboly. The forerunner cell cluster forms a bilaterally symmetric wedge at the distal margin of the advancing blastoderm. Dark nuclei are visible within the YSL. (F) At blastopore closure, the forerunner cell cluster forms the posterior limit of the embryonic axis. The embryo was followed until the tailbud stage when it was reimaged from a side view (see Fig. 5A). |
|
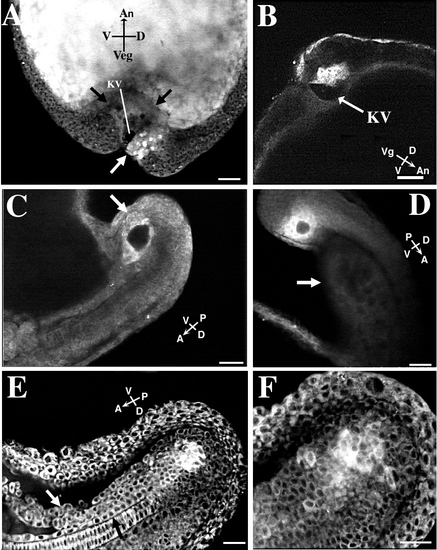
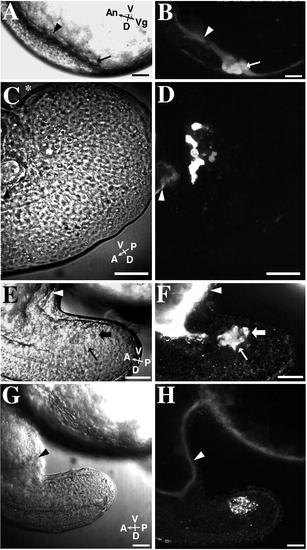
Location of forerunner cells in tail rudiment. Scale bars, 50 μm. Embryos in A, E, and F were colabeled with SYTO-11 and BODIPY 505/515 at dome stage. The embryos in B, C, and D were labeled only with SYTO-11. (A) The forerunner cell cluster with brightly stained nuclei (white arrow) in a tailbud-stage embryo is located ventral to the chordoneural hinge and germ ring on the dorsal side of the embryo. A portion of the forerunner cell cluster forms the dorsal roof of Kupffer’s vesicle (KV), the dark fluid-filled compartment. The YSL forms the ventral surface of the vesicle. The compacted YSL presses toward the animal pole into the central mass of yolk platelets within the embryo’s yolk cell (the edge of the YSL is delineated by black arrows). The forerunner cell cluster in this embryo was imaged from 70%-epiboly to blastopore closure (see Figs. 4D-4F). (B) In a tailbud-stage embryo, the forerunner cells form the dorsal roof of Kupffer’s vesicle (KV) (arrow). Side view of embryo. (C) A different embryo at the 16- to 18-somite stage with a side view of Kupffer’s vesicle surrounded by the brightly labeled (white arrow) forerunner cells. Kupffer’s vesicle is now located on the ventral side of the tail rudiment. (D) Localization of a forerunner cell cluster in an embryo at the approximately 20-somite stage. The forerunner cells surround Kupffer’s vesicle. The faintly visible yolk tube (arrow) is located on the ventral side of the extending tail. The embryo is viewed from the side, with the dorsal side of the tail facing to the top right. (E) The forerunner cell cluster remains ventral to the chordoneural hinge during early tail extension. The embryo is at approximately the 26-somite stage. Kupffer’s vesicle has disappeared by this stage of development. Side view of tail rudiment with its dorsal side facing down. (F) The same embryo as in E at a higher magnification and at a more lateral plane of focus (notochord no longer visible). Labeled cells have detached from the main forerunner cell mass. |
|
Membrane impermeant fluorescent dyes are endocytosed into the YSL and cytoplasm of NEM cells. Scale bars, 50 μm. (A) FITC-labeled dextran (10K MW) is internalized by fluid-phase endocytosis into the YSL. Numerous endosomes are distributed through the YSL. Nuclei in the YSL are unlabeled. A confocal view of the EVL–YSL margin. The embryo was labeled between oblong and sphere stage and observed at 30%-epiboly. (B) A confocal section deeper into the same embryo as shown in A. No labeling of deep cells is seen. Two EVL cells are diffusely labeled with dextran (bottom left). (C) A Nomarski view of a zebrafish embryo at 70%-epiboly. The embryo was labeled with dextran at 50%-epiboly. The characteristic thickening at the dorsal margin of the blastoderm is visible (arrow). View of the embryo from the vegetal pole. (D) Fluorescence confocal image of the same embryo as in C. Numerous fluorescently labeled endosomes are visible in the cytoplasm of the YSL and closely associated forerunner cells. (E) A Nomarski image of the blastoderm edge of an embryo, viewed from the vegetal pole, at 80%-epiboly. The forerunner cell cluster (arrow) is visible as a wedge of cells located distal to the advancing dorsal margin of the blastoderm. The embryo was labeled with fluorescent dextran at 50%-epiboly. (F) Fluorescence confocal image of the same area as shown in E. Forerunner cells possess numerous endosomes containing FITC–dextran. A sparser distribution of fluorescently labeled endosomes is visible within the YSL in the left portion of the image. |
|
Some NEM cells are in cytoplasmic confluence with the YSL in late-blastula-stage embryos. The YSL in each embryo was loaded with Texas Red-dextran (3000 MW) by impalement loading at the oblong stage of development. The embryos were then observed at later development stages. B, D, F, and H show different results obtained by these impalement experiments. A diffuse distribution of fluorescent dextran in a forerunner cell indicates that the cell was in cytoplasmic confluence with the yolk cell at the time of impalement loading. Vesicular labeling indicates that the membrane-impermeant dextran was internalized by endocytosis. Most embryos exhibited a mixture of these two staining patterns in the forerunner cells. Corresponding Nomarski (left column) and fluorescence (right column) images are shown for each embryo. Scale bar, 50 μm. (A) Nomarski image of an approximately 75%-epiboly-stage embryo at the dorsal margin. The embryo is viewed from the side with its dorsal aspect facing down. The black arrow and arrowhead point to the locations of the forerunner cell cluster and YSL respectively, and correspond to those locations seen in B. (B) Some forerunner cells (arrow) are diffusely labeled with fluorescent dextran. Note the bright, diffusely labeled YSL (arrowhead). (C) An embryo’s tail viewed at the 14-somite stage from the side. The dorsal aspect of the tail is toward the bottom of the image. The most posterior part of the curved tail is marked by an asterisk (*). The black arrowhead points to the YSL and posterior part of the yolk tube and corresponds to the arrowhead in D. (D) This image shows an embryo with diffuse-dextran labeling in only a few of the forerunner cells. (E) Nomarski image of a tail of a 16-somite-stage embryo viewed from the side. The arrows correspond to the location of the arrows shown in F. (F) Forerunner cells exhibit both diffuse (thin arrow) and vesicular (thick arrow) fluorescent – dextran labeling. Forerunner cells are no longer in contact with the YSL (white arrowhead). (G) Nomarski image of a 16-somite-stage embryo in the same orientation as E and F but at a lower magnification. (H) The forerunner cells in this embryo show prominent vesicular labeling. Diffuse dextran labeling is present in the YSL (white arrowhead), which extends into the yolk tube at the base of the tail rudiment. |
|
The NEM cell cluster is composed of a mixture of cells, some of which are connected to the YSL in oblong-stage embryos. In three different embryos (A and B, C and D, E and F), the yolk cell was loaded with Texas Red-dextran at the oblong stage of development. The embryos were then allowed to internalize SYTO-11 by endocytosis at dome stage. The embryos were observed at 70%-epiboly (A-D) or at 14-somite stage (E, F). The top row shows SYTO-11 labeling (A, C, E); the bottom row shows Texas Red-dextran labeling (B, D, F). (A, B) NEM/forerunner cells, labeled with SYTO-11, are located at the dorsal side of the blastoderm. Thick arrows point to cells that also contain Texas Red-dextran (see B). These cells are located near the YSL-EVL junction. Thin arrows point to SYTO-11 labeled NEM/forerunner cells that do not contain Texas Red-dextran (see B). This result indicates that SYTO-11 can accumulate in NEM/forerunner cells in the absence of a cytoplasmic connection to the YSL. (C, D) A different embryo shows a similar mixture of labeled and unlabeled cells. Thick arrows point to SYTO-11-labeled NEM/forerunner cells containing Texas Red-dextran. Thin arrows point to NEM/forerunner cells that do not contain Texas Red-dextran. (E, F) A 14-somite-stage embryo viewed from the side with Kupffer’s vesicle visible. The thick arrow points to a forerunner cell that contains Texas Red-dextran; the thin arrow points to a forerunner cell that is devoid of Texas Red-dextran. |
Reprinted from Developmental Biology, 180(1), Cooper, M.S. and D'Amico, L.A., A cluster of noninvoluting endocytic cells at the margin of the zebrafish blastoderm marks the site of embryonic shield formation, 184-198, Copyright (1996) with permission from Elsevier. Full text @ Dev. Biol.