- Title
-
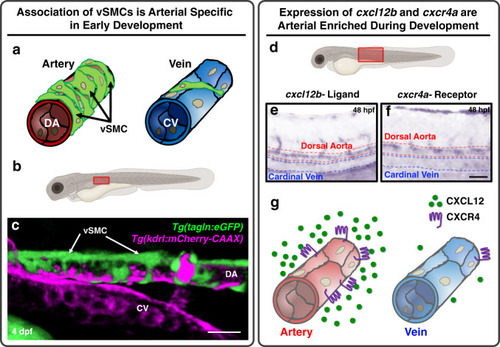
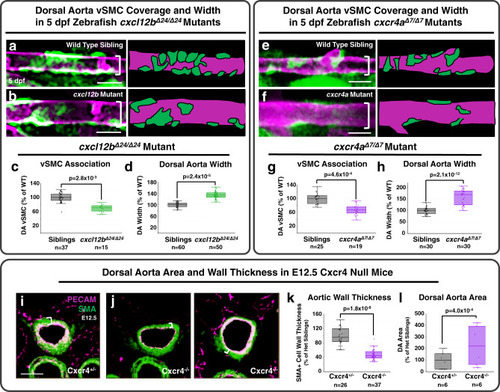
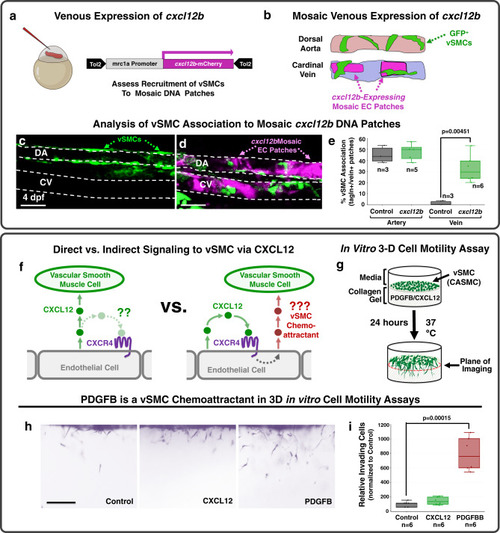
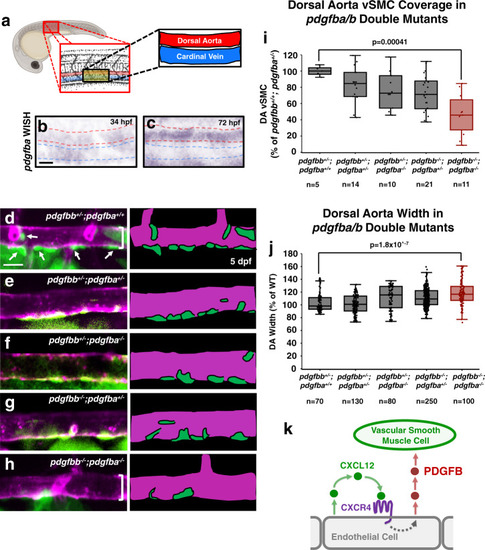
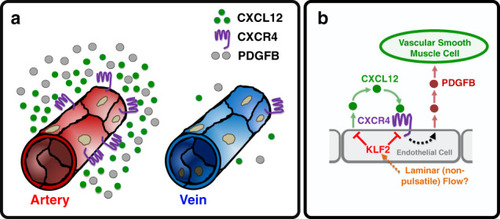
Chemokine mediated signalling within arteries promotes vascular smooth muscle cell recruitment
- Authors
- Stratman, A.N., Burns, M.C., Farrelly, O.M., Davis, A.E., Li, W., Pham, V.N., Castranova, D., Yano, J.J., Goddard, L.M., Nguyen, O., Galanternik, M.V., Bolan, T.J., Kahn, M.L., Mukouyama, Y.S., Weinstein, B.M.
- Source
- Full text @ Commun Biol
|
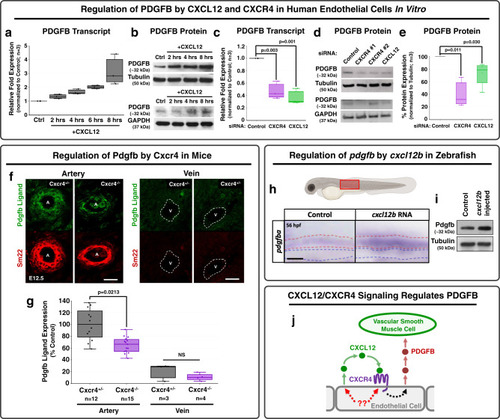
EXPRESSION / LABELING:
|
|
PHENOTYPE:
|
|
|
|
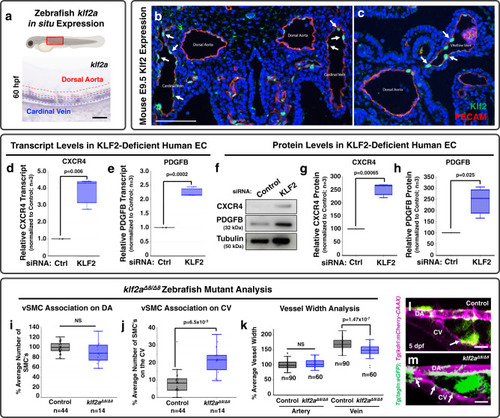
EXPRESSION / LABELING:
PHENOTYPE:
|
|
EXPRESSION / LABELING:
|
|
|
|
|