|
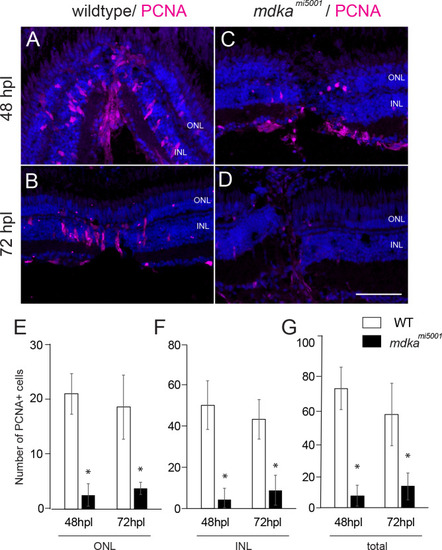
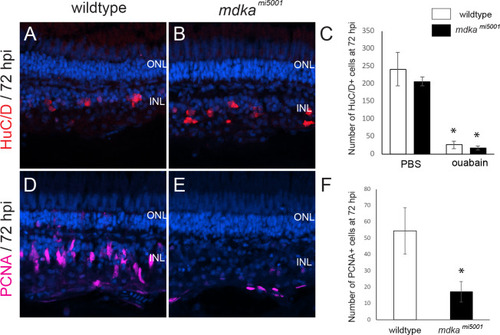
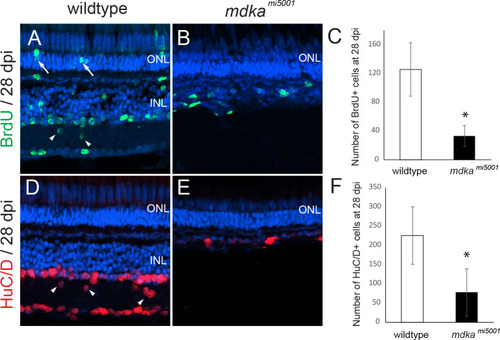
Proliferation of Müller glia is compromised following a stab wound in <italic>mdka</italic><sup><italic>mi5001</italic></sup>.(A-D) Immunocytochemistry for proliferative marker, PCNA, in wildtype (A,B) and mdkami5001 (C,D) at post-lesion time points. (E-G) Graphs illustrating the number of PCNA-positive cells in the outer nuclear layer (E; 48 hpl, wildtype: 20.9 ± 3.7 cells, mdkami5001: 2.6 ± 2.1 cells, p = 0.0016; 72 hpl, wildtype: 18.5 ± 5.8 cells, mdkami5001: 3.8 ± 1.1 cells, p = 0.0126), inner nuclear layer (F; 48 hpl, wildtype: 50.7 ± 11.9 cells, mdkami5001: 4.4 ± 5.3 cells, p = 0.0036; 72 hpl, wildtype: 43.7 ± 9.7 cells, mdkami5001: 8.9 ± 7.3 cells, p = 0.0077), and total retinal layer (G; 48 hpl, wildtype: 72.1 ± 12.9 cells, mdkami5001: 7.1 ± 6.3 cells, p = 0.0014; 72 hpl, wildtype: 56.4 ± 19.1 cells, mdkami5001: 12.8 ± 8.0 cells, p = 0.0212). Scale bar equals 60 μm. Student’s t-test, p*<0.05, n = 3 wildtype and 3 mutants. ONL: outer nuclear layer; INL: inner nuclear layer.
|