- Title
-
Apcdd1 is a dual BMP/Wnt inhibitor in the developing nervous system and skin
- Authors
- Vonica, A., Bhat, N., Phan, K., Guo, J., Iancu, L., Weber, J.A., Karger, A., Cain, J.W., Wang, E.C.E., DeStefano, G.M., O'Donnell-Luria, A.H., Christiano, A.M., Riley, B., Butler, S.J., Luria, V.
- Source
- Full text @ Dev. Biol.
|
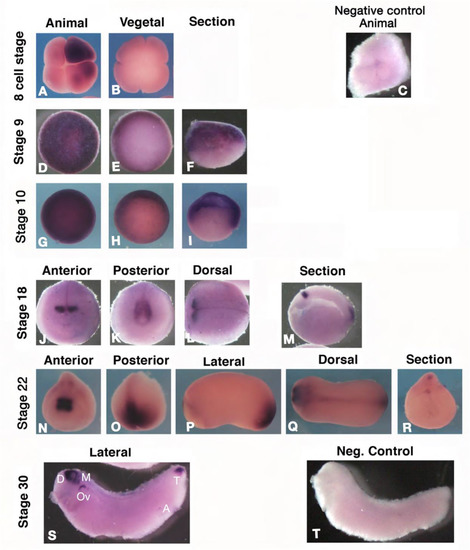
Expression of Xenopus apcdd1. A-T. RNA in situ hybridization for Xenopus apcdd1. Maternal expression at 8 cell stage (A, B) is localized in the animal pole and is frequently unevenly distributed. In late blastulas (stage 9, D-F), the expression is animal and marginal (arrows in F, blastocoel in outlined in F). At the start of gastrulation (stage 10, G-I) apcdd1 RNA is present in animal and marginal cells, including the dorsal lip with the Spemann organizer (arrow in H, I, blastocoel is outlined in I). In neurula embryos (stage 18, K-N) and tailbud (stage 22), expression is limited to diencephalon and midbrain precursors (J, K, M, N, Q), and the tailbud (K, M, O, P, Q). Arrowhead in the transversal section (R) indicates ependymal precursors. In tadpoles (stage 30, S) expression closely mirrors that in mouse embryos. D – diencephalon; M – midbrain; Ov – otic vesicle; T – tailbud; A – anus. Bracket in S indicates the branchial arches. For control in situ hybridization (C: 8-cell stage; T: tadpole), the RNA probe used was LacZ. The scale bar in A is 0.3 mm (all embryos are shown at the same scale). At least 10 Xenopus embryos were examined for each time point. |
|
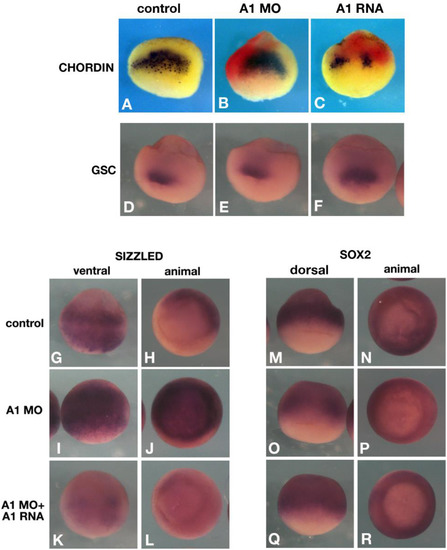
Apcdd1 regulates expression of both positive and negative targets of BMP signaling in Xenopus embryos. In situ hybridization of morpholino-depleted (A1MO), or Apcdd1 overexpressing embryos. Embryos were injected at the 4-cell stage dorsally (A-C, double in situ hybridization for chordin and LacZ RNA as injection marker, in red), or 1 cell stage (D–R). Control embryos were injected solely with LacZ RNA (1 ng). A-F. Spemann organizer genes: chordin is not affected by apcdd1 depletion (B) (n = 18 embryos), but inhibited by apcdd1 RNA (A1 RNA) overexpression (n = 23 embryos), consistent with the inhibition of the maternal, dorsal Wnt pathway; gsc is unaffected by either treatment (n = 15 and n = 12 embryos, respectively, E, F). G-L. The ventral marker and BMP target sizzled is expanded by Apcdd1 depletion (n = 23, I, J), and restored by expression of a MO-resistant apcdd1 RNA (n = 21 embryos, K, L). M-R. The early neural marker and negative BMP target sox2 is inhibited by Apcdd1 depletion (n = 27 embryos, O, P, arrows in O indicate a hole in the ventral expression pattern), and partially restored by expression of Apcdd1 RNA (n = 17 embryos, Q, R). |
|
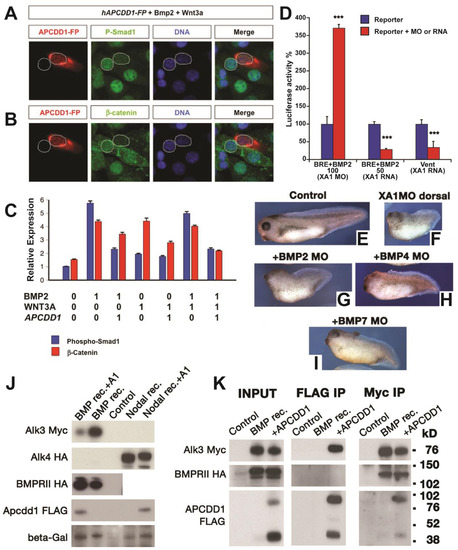
APCDD1 inhibits BMP signaling in cells and tissues. A-B. The activation of BMP and Wnt pathways was measured at single-cell level, using NIH 3T3 mouse fibroblast cells cultured in the presence of BMP and Wnt ligands. Cells transfected with human APCDD1 and a fluorescence reporter (displayed in red) have low levels of both pSmad1 (green in A) and β-catenin (green in B), while neighboring cells that are not transfected have higher levels of both effectors. Nuclei are visualized with DAPI (blue). C. The immunofluorescence levels of pSmad1 and β-catenin were measured relative to the background (relative expression, Y axis), in the presence (0) or absence (1) of one or both ligands (Bmp2, Wnt3A) or of APCDD1 (n = 40 cells per condition). The outputs of BMP and Wnt signaling are inhibited by APCDD1 (p < 0.001 in Bonferroni-adjusted 2-way heteroscedastic t-test comparisons). D. Effect of Xenopus Apcdd1 (XA1) modulation on BMP-induced transcriptional activity in Xenopus animal injections. Apcdd1 depletion increases transcription induced by exogenous BMP2 RNA, while overexpression of apcdd1 RNA reduces transcription from both the BRE reporter induced by exogenous BMP2, and of the Vent promoter induced by endogenous BMP ligands. ∗∗∗p < 0.001 in Student’s t-test. Embryos were collected at stage 10.5, four embryos per assay, in triplicate, and each experiment was repeated a minimum of 3 times. E-I. The phenotype of XA1 depletion on the dorsal side is rescued most effectively by simultaneous BMP7 depletion (quantification in Fig. S1E). Embryos injected at the 4-cell stage on the dorsal side were collected at stage 36. The dorso-anterior index (DAI) values (Kao and Elinson, 1988) are: E – DAI5 (normal), F – DAI2, G – DAI2, H – DAI3, I – DAI4. For D-I, at least 10 Xenopus embryos, in three different experiments, were examined for each time point and experimental condition. J. Human APCDD1 specifically reduces the protein level of type I BMP receptor BMPRIA, but not the type II receptor BMPR2 and nodal type I receptor ACVR1B. Beta-galactosidase serves as loading control. Xenopus embryos were injected in the animal poles. K. APCDD1 interacts directly with BMPRIA, but not with BMPR2, in CHO cells. APCDD1 and BMPRIA coprecipitated in both APCDD1 (center panel) and BMPRIA (right panel) immunoprecipitation. BMPR2 coprecipitated with BMPRIA (right panel), but not with APCDD1 (center panel). |
|
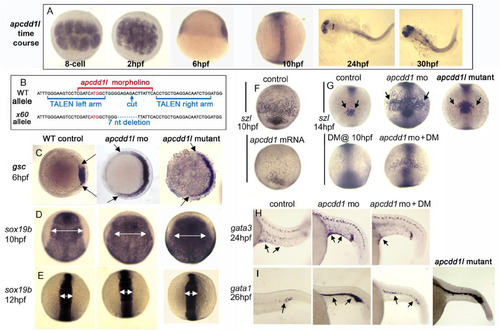
Role of APCDD1 in zebrafish axis elongation and dorso-ventral patterning. A. Expression of apcdd1l in zebrafish embryos at the indicated times. The presence of maternal apcdd1l mRNA, confirmed by RT-PCR (not shown), is seen in all cells in the early blastula (animal pole views). By early gastrulation (6 hpf), apcdd1l is only weakly expressed with preferential staining in dorsal tissues (lateral view, dorsal to the right). By the end of gastrulation (10 hpf), apcdd1l is expressed throughout to the dorsal axis (dorsal view and lateral view, anterior up). At 24 and30 hpf, apcdd1l is expressed in the eyes, restricted regions in the brain, and dorsal tissues in the trunk and tail. B. Partial sequence of exon 1 surrounding the initiation codon (red font) showing the binding sites for apcdd1l-mo (red font) and TALEN left and right arms (blue font) used to induce double strand breaks. The induced x60 mutant allele has a 7 nucleotide deletion, leading to a frame shift followed by 18 premature stops in exon 1 (a presumptive null). C. Expression of the organizer gene goosecoid (gsc) at 6 hpf (early gastrula stage). Knockdown of apcdd1l, or loss of apcdd1l in MZmutants, disinhibits maternal Wnt, resulting in expansion of the organizer. Animal pole views with dorsal to the right. D-E. Expression of neural plate marker sox19 b at the end of gastrulation (C) and at the 6-somite stage (D). The early expansion of gsc in apcdd1-morphants and MZ mutants results in a dorsalized phenotype that persists through the end of gastrulation, but dorsalization is rapidly reversed by early somitogenesis stages. Dorsal views with anterior to the top. F. Expression of the Bmp feedback inhibitor sizzled (szl) in ventral ectoderm (anterior up) at the end of gastrulation. Expression of szl marks cells experiencing active Bmp signaling. Injection of apcdd1l mRNA into wild-type embryos at the one-cell stage results in downregulation of szl by the end of gastrulation. G. Expression of szl in ventral ectoderm (anterior up) at the 10-somite stage. apcdd1l-morphants and MZmutants exhibit an expanded ventral domain of szl, indicating that Bmp signaling is now higher than normal. Treatment of embryos from 10 hpf with 75 μM dorsomorphin (DM) abolishes szl expression in control embryos and partially reverses expansion of szl expression in apcdd1l-morphants. H-I. Expression of gata3 in ventral epidermis ionocytes (arrows) at 24 hpf (G) and gata1 in blood progenitors (arrows) at 27 hpf (H). Ventral ionocytes and blood progenitors are expanded in apcdd1l-morphants, indicating ventralization of caudal structures. MZapcdd1l mutants also show an expanded domain of gata1. Treatment of apcdd1l-morphants with 75 μM DM from the end of gastrulation partially reverses ventralization. Images show lateral views with anterior to the left.At least n = 15 zebrafish embryos were examined for each time point and experimental condition. |
|
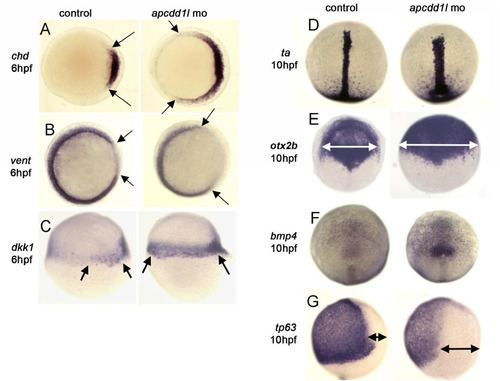
Expression of zebrafish apcdd1 and effects of functional knockdown on embryonic patterning. A-G. Domains of expression of various markers of gastrula-stage embryos. Expression of chd in the organizer and vent in ventrolateral mesoderm (A, B, animal pole view) and dkk1 in the organizer (C, lateral view). The organizer is expanded laterally at 6 hpf in apcdd1l morphants, and there is a corresponding contraction of ventrolateral tissue. At 10 hpf, expression of brachyury-a (ta) in the notochord (D) and otx2b in the anterior neural plate (E) (dorsal views, anterior up) are expanded in apcdd1l morphants, whereas expression of ventral marker tp63 in epidermal ectoderm (G, lateral view) is contracted. At this time, expression of bmp4 at the ventral midline (F, dorsal view) begins to upregulate and expand in apcdd1l morphants, presaging subsequent ventralization. At least n = 15 zebrafish embryos were examined for each time point and experimental condition. |
|
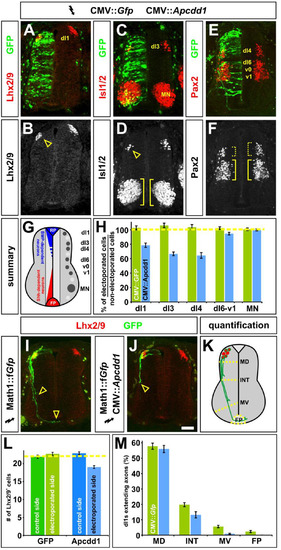
Mis-expression of Apcdd1 blocks BMP-specific activities in the dorsal spinal cord. A-F, H. Gfp alone (control, H), or Gfp (green) in combination with APCDD1 (experimental, A-F, H), was ectopically expressed throughout the spinal cord under the control of the CMV enhancer by in ovo electroporation at Hamilton Hamburger (HH) stages 14/15. Embryos were harvested at HH stage 23 and examined for the number of Lhx2/9+ dI1 (commissural) neurons (red, A, B), Isl1/2+ dI3 and motor neurons (MNs) (red, C, D) or Pax2+ dI4 and dI6-v1 neurons (red, E, F). G. The relative positions of the labeled post-mitotic neurons are shown schematized within the spinal cord. The dorsal most populations of spinal neurons (dI1-dI3) are known to be dependent on BMP signaling, the role of BMP signaling in the more ventral-dorsal populations (dI4-dI6) has remained unresolved. H. Quantification revealed that the misexpression of Gfp (n = 42 sections from 6 embryos) had no significant effect on any of the monitored populations of spinal neurons (dI1, p > 0.64 compared to the non-electroporated control side of the same embryo; dI3, p > 0.29; dI4, p > 0.44; dI6-v1, p > 0.40; MN, p > 0.49). In contrast, the ectopic expression of both Gfp and Apcdd1 (n = 35 sections from 6 embryos) resulted in a 25–35% loss in BMP-dependent dorsal neural populations: dI1 neurons (p < 0.03) dI3 neurons (p < 0.008) and dI4 neurons (p < 3.5 × 10−6). However, APCDD1 had no detectable effect on the numbers of BMP-independent neurons, specifically dI6, vo, v1 interneurons (p > 0.055) and MNs (p > 0.54). I. Lhx2/9+ dI1 neurons (red) electroporated with farnesylated (f) Gfp under the control of the Math1 (Atoh1) enhancer (Math1::fGfp) at HH stages 16/17, GFP+ axons (green) are extending into the ventral spinal cord by HH stage 22, with a few axons having reached the floor plate (FP, arrowheads). J. In contrast, Lhx2/9+ dI1 neurons (red) concomitantly electroporated with both Math1::fGfp (green) and CMV:: Apcdd1 showed over an 80% reduction of axon outgrowth at the same stage, with no axons reaching the FP (arrowhead). K. The extent of the dI1 axon outgrowth was quantified by determining whether dI1 axons had crossed one of four arbitrary lines in the spinal cord: mid-dorsal (MD), intermediate (INT), mid-ventral (MV) or the FP, as in (Phan et al., 2010). L. Misexpression of Apcdd1 throughout the spinal cord at HH stage 16/17 has a small but significant effect on the numbers of dI1 neurons. There is a 10% reduction in dI1 neurons on the APCDD1-electroporated side (p < 2.2 × 10−9, n = 173 sections from 6 embryos) compared to the control Gfp electroporated side (n = 178 sections from 5 embryos). M. By HH stage 22, approximately 55% of Lhx2/9+ neurons electroporated with gfp (control) or gfp with APCDD1 (experimental) have extended GFP+ axons. In control embryos (n = 178 sections, taken from 5 embryos), 10% of these axons extended to the MV line and 4% reached the FP. In contrast, only 1.6% of Lhx2/9+ neurons in the embryos electroporated with both Gfp and Apcdd1 (n = 173 sections, taken from 6 embryos) had extended to axons to the MV line (p < 0.0001, similar to GFP controls) with none reaching the FP (p < 0.02). Statistical analyses were performed using a one-tailed Student’s t-test, when the data fit a normal distribution, and with the Mann–Whitney test. Scale bar: 25 μm. |
|
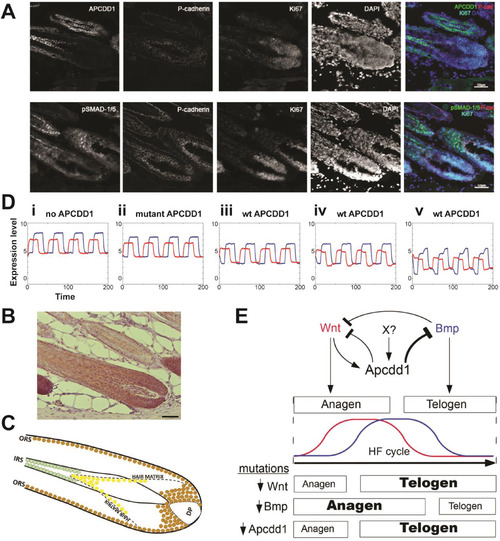
APCDD1 protein is expressed in mouse hair follicles and can coordinate hair follicile dynamicsA. Immunofluorescence staining of adult mouse hair follicle cryosections detects expression of APCDD1 (upper row) and respectively pSmad1/5 (lower row) in relation to hair follicle matrix marker P-cadherin and proliferation marker Ki67. The two sections are 20 μm apart. Nuclei are visualized with DAPI. Stains were performed on 6 mice, having 7-8 sections per mouse. B. In mouse hair follicles paraffin sections, activated β-catenin (brown) is present in the hair matrix abutting the dermal papilla (DP) and in the outer root sheet (ORS). Scale bar: 50 μm. Stains were performed on 6 mice, having 7-8 sections per mouse. C. Schematic of immunodetection results. Activated β-catenin (brown), an effector of Wnt signaling, is localized along the outer root sheath (ORS) and hair bulb, surrounding the dermal papilla (DP) that produces the growth signals during anagen. Nuclear pSMAD-1/5 (yellow), an effector of BMP signaling, is located in the distal portion of the hair matrix, where keratinocytes begin to differentiate into the layers of the inner root sheath (IRS). APCDD1 expression (green) is localized to the mature keratinocytes of the IRS, consistent with its function to downregulate both Wnt and BMP signaling. D. Mathematical model of the hair follicle temporal activation dynamics of the BMP and Wnt pathways (pSmad1 in blue, β-catenin in red). The BMP and Wnt ligand inputs are periodic, have the same period and are out of phase by a quarter of a period. Cases shown: (i) no APCDD1 is present, as in null mutants; (ii) mutant APCDD1, which is a very weak inhibitor (Shimomura et al., 2010); (iii) wild type APCDD1, which inhibits Wnt signaling (Shimomura et al., 2010) and BMP signaling; (iv) wild type APCDD1, and added moderate repression of Wnt signaling by BMP signaling; (v) wild type APCDD1, added moderate repression of Wnt signaling by Bmp signaling, and Apcdd1 is induced by Wnt signaling. The absence of Apcdd1 allows higher levels of pSmad1; wild type Apcdd1 depresses pSmad1 levels. E. APCDD1 may control the duration of the phases of the hair follicle cycle. Summary of Apcdd1 activity: First, APCDD1 expression is induced by Wnt signaling and also by a currently unidentified factor X, which maintains APCDD1 after Wnt signaling ceases. Second, APCDD1 represses both BMP signaling and Wnt signaling (which also is repressed by BMP signaling). Bmp and Wnt signaling together control the duration of hair follicle phases anagen (when hair grows) and telogen (when hair falls out). Third, mutations inactivating Wnt signaling, or Bmp signaling, or APCDD1, result in changed durations of anagen and telogen. |
Reprinted from Developmental Biology, 464(1), Vonica, A., Bhat, N., Phan, K., Guo, J., Iancu, L., Weber, J.A., Karger, A., Cain, J.W., Wang, E.C.E., DeStefano, G.M., O'Donnell-Luria, A.H., Christiano, A.M., Riley, B., Butler, S.J., Luria, V., Apcdd1 is a dual BMP/Wnt inhibitor in the developing nervous system and skin, 71-87, Copyright (2020) with permission from Elsevier. Full text @ Dev. Biol.