- Title
-
Size control of the inner ear via hydraulic feedback
- Authors
- Mosaliganti, K.R., Swinburne, I.A., Chan, C.U., Obholzer, N.D., Green, A.A., Tanksale, S., Mahadevan, L., Megason, S.G.
- Source
- Full text @ Elife
|
( |
|
( |
|
( |
|
( |
|
( |
|
( |
|
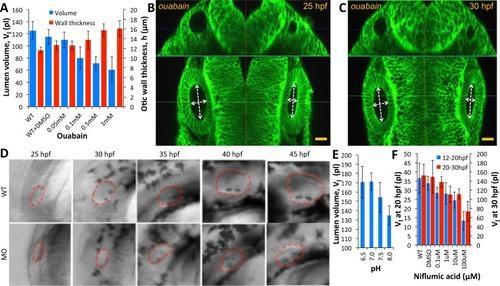
In addition to experiments at 30 hpf reported in |
|
In addition to experiments at 30 hpf reported in |
|
2D+time confocal movie showing growth and regeneration inhibition from ouabain treatment. Labelling by ( |
|
( |
|
( |