- Title
-
Preservation of zebrafish genetic resources through testis cryopreservation and spermatogonia transplantation
- Authors
- Marinović, Z., Li, Q., Lujić, J., Iwasaki, Y., Csenki, Z., Urbányi, B., Yoshizaki, G., Horváth, Á.
- Source
- Full text @ Sci. Rep.
|
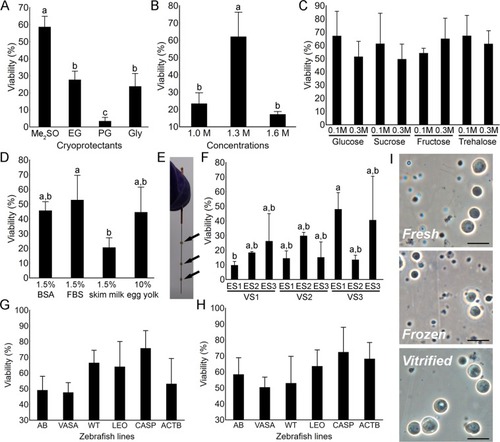
Optimization of the slow-rate freezing ( |
|
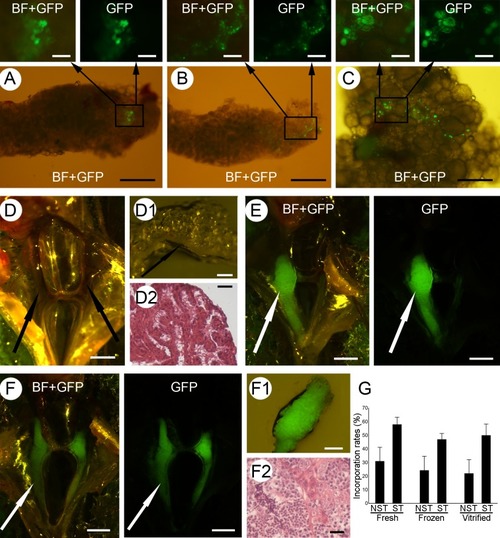
Incorporation and proliferation of fresh and cryopreserved spermatogonia. The incorporation and proliferation of fresh ( |
|
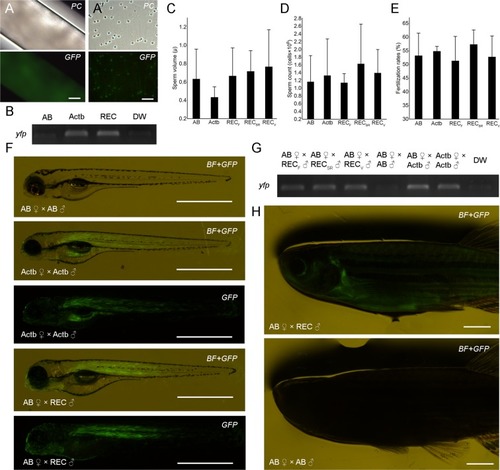
Production of donor-derived spermatozoa and progeny from MO-sterilized recipients. Milt ( |