- Title
-
Fibroblast growth factor signaling mediates progenitor cell aggregation and nephron regeneration in the adult zebrafish kidney
- Authors
- Gallegos, T.F., Kamei, C.N., Rohly, M., Drummond, I.A.
- Source
- Full text @ Dev. Biol.
|
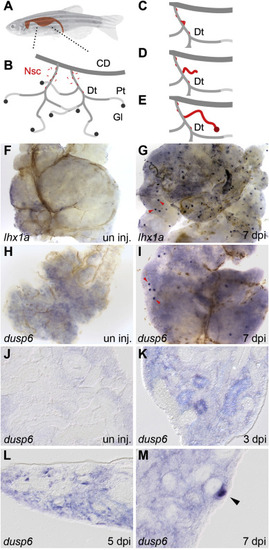
lhx1a and dusp6 expression marks new nephron formation in the regenerating adult zebrafish kidney. (A) An adult zebrafish with the shape of the kidney highlighted. (B) Diagram of the organization of the adult kidney. Nephrogenic stem cells (Nsc) are colored red and appear in the interstitium and along the renal tubules. Segments of the kidney tubular structure are: CD, collecting duct; Dt, distal tubule; Pt, proximal tubule, Gl, glomerulus. (C–E) Diagram of stem cell based nephrogenesis. Stem cells accumulate on the Dt segment and form aggregates of cells (C). The aggregate elongates into a tubular epithelium and connects to the Dt (D). The new nephron fully differentiates to form a functional nephron (E). (F) Whole mount in situ hybridization of control uninjured adult kidneys show no expression of lhx1a. (G) Seven days after gentamicin injury lhx1a was induced in aggregates of nephrogenic cells, red arrowheads. (H) dusp6 was not expressed in controls but was induced in nephrogenic cells and cell aggregates at 7 dpi, red arrowheads (I). Scale Bars F–I are 500 μm. (J) In histological sections, dusp6 was not expressed in uninjured control kidneys. J-M empty arrowheads indicate dusp6 negative tubules (K) dusp6 was induced by kidney injury in the interstitium and some renal tubules at 3 dpi, in aggregating single cells adjacent to kidney tubules at 5 dpi (L), and highly expressed in nephrogenic aggregates at 7 dpi (M). In K-M, representative dusp6 expressing structures are indicated with solid arrowheads. Scale Bars J-M are 50 μm. EXPRESSION / LABELING:
|
|
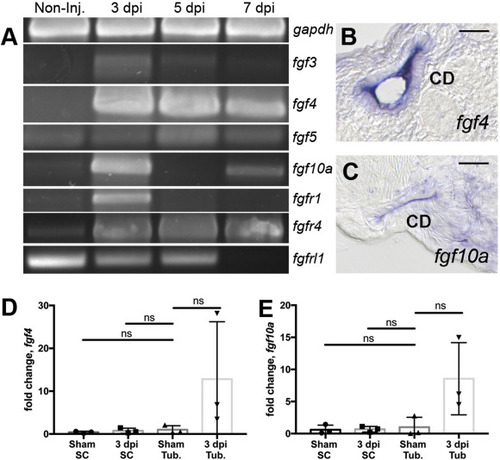
RT-PCR analysis identifies injury modulated FGF signaling molecules. (A) RTPCR assays revealed that fgf3, fgf4, fgf5, fgf10a and the receptors fgfr1, fgfr4 were induced post-injury while the kinase domain deficient fgfrl1 gene exhibited reduced expression after injury. Histological sections of whole mount in situ hybridization 7 dpi injured kidneys show fgf4 (B) and fgf10a (C) were expressed in the collecting duct epithelium. (B–C) Scale bars are 20 μm In kidney cell fractionation experiments, fgf4 (D) and fgf10 (E) were induced in isolated tubules and not in single interstitial cells at 3dpi compared to sham injured kidneys. qPCR represents 3 biological replicates. Comparisons were made by Kruskal-Wallis test. ns indicates not-significant. Data are expressed as mean ± SD. |
|
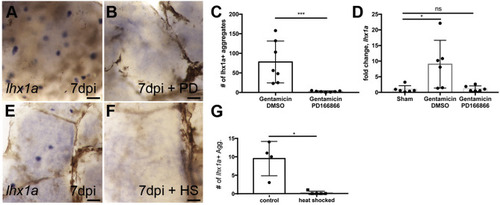
Inhibition of Fgf signaling prevents new nephron development. (A) Injured zebrafish generate lhx1a + nephrogenic aggregates at 7 dpi by whole mount in situ hybridization. (B) Treatment with the FGFR1 inhibitor PD166866 blocks the appearance of lhx1a + nephrogenic aggregates in whole mount kidneys. (C) Quantification of lhx1a + nephrogenic aggregates in multiple whole mount kidneys demonstrates PD166866 completely blocks kidney regeneration. N = 7 fish per condition. Error bars indicate ±SD. p = 0.01 by Mann-Whitney T-test. (D) qRTPCR quantitation of lhx1a expression shows PD166866 treatment blocks lhx1a expression post-injury. N = 6 fish per condition. Error bars indicate ±SD. * indicates p = 0.02 by Kruskal-Wallis test. (E) Control non-heat shocked Tg(hsp70:dnfgfr1) transgenic fish show lhx1a + new nephron aggregates 7 days following gentamicin injury. (F) Heat shock induction of the dominant-negative Fgfr1 receptor blocked the development of lhx1a + new nephron aggregates. (G) Quantification of lhx1a + nephrogenic aggregates in multiple whole mount kidneys demonstrates that expression of the dominant-negative Fgfr1 receptor completely blocks kidney regeneration. Differences in injury responses (number of nephrogenic aggregates in A vs E) reflect strain dependent differences in sensitivity to gentamicin injury. N = 4 control and N = 5 heat shocked fish. Error bars indicate ±SD. p = 0.03 by Mann-Whitney T-test. In panels A,B,E,F scale bars are 100 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
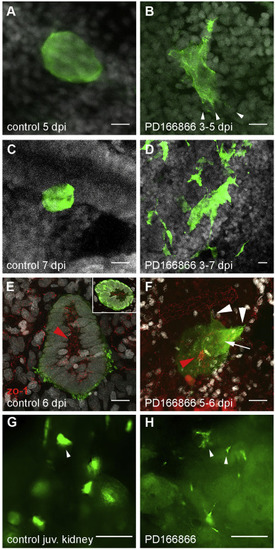
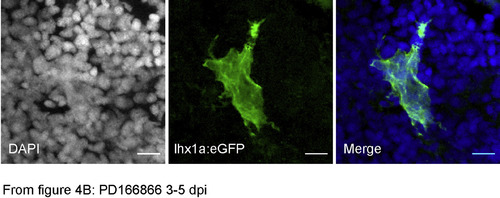
Disruption of nephrogenic cell aggregate formation in Fgf signaling inhibited kidneys. (A) Adult Tg(lhx1a:EGFP) fish at 5 dpi exhibit condensed, dome-shaped cell aggregates on distal tubules. (B) Treatment of regenerating fish from 3 to 5 days post injury with the Fgfr1 inhibitor PD166866 followed by imaging at 5 dpi showed delayed formation of tight adherent nephrogenic cell domes and the appearance of cell lamellopodia (arrowheads in B). (C–D) Tight, condensed lhx1a:EGFP + nephrogenic cell aggregates seen in control injured kidneys (C) did not form in fish treated with PD166866 from 3 to 7 dpi (D); instead lhx1a:EGFP + cells remained stellate-shaped and disorganized. (E) 6 dpi lhx1a:EGFP + nephrogenic cell aggregates display a rosette shape with a zo-1 positive apical lumen (inset) extending in the direction of nephron growth (red arrowhead; proximal). (F) 24 h PD166866 treatment from day 5–6 induces filopodia and lamellopodia formation (white arrowheads) and reduction in apical zo-1 localization (white arrow) with a ring of remnant zo-1 only in distal cells (red arrowhead). (G) Juvenile Tg(lhx1a:EGFP) fish at 42 dpf produce nephrogenic aggregates in their developing kidneys identical to injury-induced, dome-shaped aggregates (arrowhead in G). (H) Treatment of juveniles from 35 to 42 dpf with PD166866 results in the loss of domed aggregates and appearance of single cells and stellate cell clusters (arrowheads in H), indicating a loss of adhesion. Scale bars in Z-projections (A–F) are 10 μm and 100 μm in (G,H). PHENOTYPE:
|
|
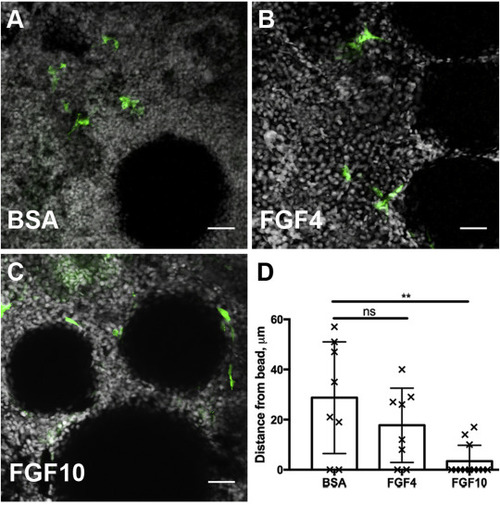
Exogenous FGF beads are sufficient to recruit progenitor cells. A) Stem cells marked by the transgene lhx1a:eGFP are not attracted to BSA soaked agarose beads after 24 h of culture. (B) Beads soaked with Fgf4 are more closely surrounded by renal stem cells, indicated by a decrease in cell to bead distance from an average of 28.8 μm–17.75 μm. (C) Beads soaked with FGF10 strongly attract renal stem cells which accumulate adjacent to the bead at a mean distance of 3.42 μm. (D) Quantification of cell to bead distances after 24 h of culture demonstrate a chemoattractive role of FGF ligands on the recruitment renal stem cells. 3 biological replicates per condition. Error bars indicate ±SD. Comparisons to BSA beads were made by Kruskal-Wallis test. ns indicates not-significant, starred comparison indicates p = 0.008. In Z-projection panels (A–C) scale bar is 25 μm. |
|
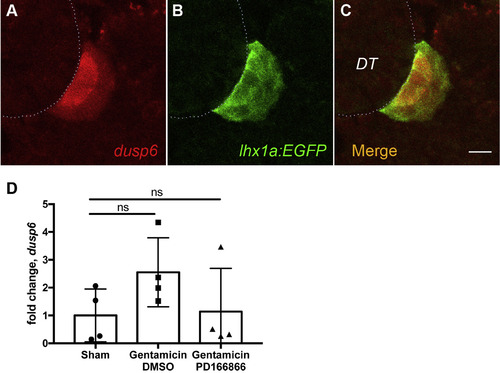
Dusp6 expression in nephrogenic cell aggregates. (A) Immunostaining with anti-Dusp6 (red) co-localizes with anti-GFP immunostaining of the lhx1a:eGFP transgene (B) at 7 dpi. (C) Merged image of A and B. Dotted line represents outline of adjacent distal tubule. Scale bars are 10 μm. (D) Injury-induced expression of dusp6 is reduced by PD166866 (added to tank water from 1 dpi to 7 dpi) to sham injured levels. qPCR represents N = 4 fish per condition. All comparisons not significant by Kruskal-Wallis test. |
|
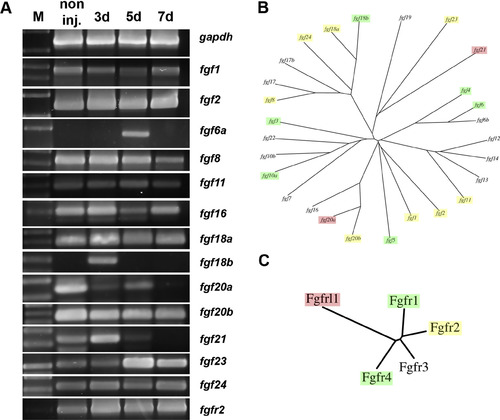
RT-PCR screen of FGF family genes in regenerating kidneys. (A) RT-PCR analysis of FGF ligand and receptor gene expression reveals additional changes in gene expression following injury as well as many Fgf family member mRNAs that remain unchanged after AKI. (B) Dendrogram of the FGF ligands indicating genes that were injury induced (green), repressed (red), or unchanged (yellow). (C) Dendrogram of the FGF receptors indicating genes that were injury induced (green), repressed (red), or unchanged (yellow). Fgf family genes that showed no kidney expression in the RT-PCR screen are not shown. |
|
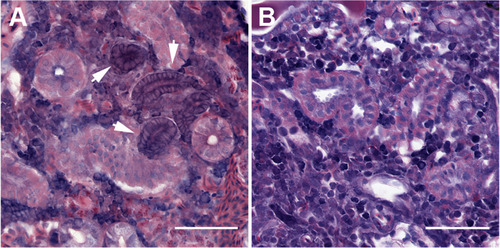
FGF signaling is required for aggregation of cells into nephrogenic condensates. (A) Nephrogenic aggregates are apparent in histological sections as compact basophilic structures adjacent to distal tubules at 7 dpi. Arrows indicate nascent nephrons. (B) FGF inhibition with PD166866 post-injury prevents the formation of nephrogenic aggregates, and no peri-tubular basophilic cellular aggregates can be found in histological sections. Scale bars are 50 μm. |
|
Channel separated and merged image of Fig. 4B. To better resolve nuclei and GFP + cell scale, Fig. 4B is represented as separate color channels DAPI, eGFP and the merged image. |
Reprinted from Developmental Biology, 454(1), Gallegos, T.F., Kamei, C.N., Rohly, M., Drummond, I.A., Fibroblast growth factor signaling mediates progenitor cell aggregation and nephron regeneration in the adult zebrafish kidney, 44-51, Copyright (2019) with permission from Elsevier. Full text @ Dev. Biol.