- Title
-
A transgenic zebrafish line for in vivo visualisation of neutrophil myeloperoxidase
- Authors
- Buchan, K.D., Prajsnar, T.K., Ogryzko, N.V., de Jong, N.W.M., van Gent, M., Kolata, J., Foster, S.J., van Strijp, J.A.G., Renshaw, S.A.
- Source
- Full text @ PLoS One
|
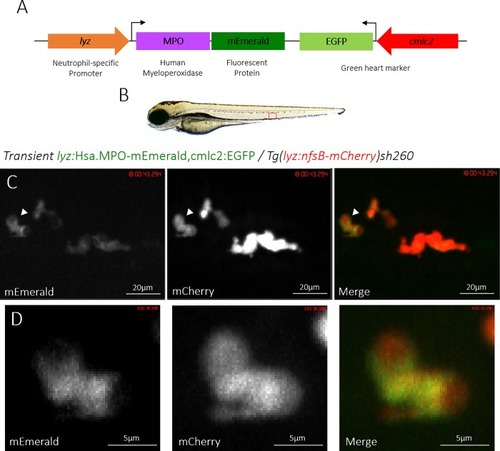
Transient expression of the lyz:MPO-mEmerald transgene labels zebrafish neutrophils. A) Schematic of the lyz:MPO-mEmerald cmlc2:EGFP construct, which includes the neutrophil-specific promoter (lyz), the MPO gene with a C-terminal fluorescent tag (MPO-mEmerald) and a green heart marker to aid optimisation of transgenesis (cmlc2:EGFP). B) A zebrafish larva at 3 days post fertilisation (dpf), with the caudal haematopoietic tissue (CHT) indicated by the red box. C) The CHT of a double-transgenic Transient lyz:MPO-mEmerald,cmlc2:EGFP; Tg(lyz:nfsB-mCherry)sh260 larva with a population of neutrophils expressing both mEmerald and mCherry. The white arrowhead indicates the neutrophil enlarged below. D) Enlarged view of a neutrophil expressing mEmerald and mCherry. |
|
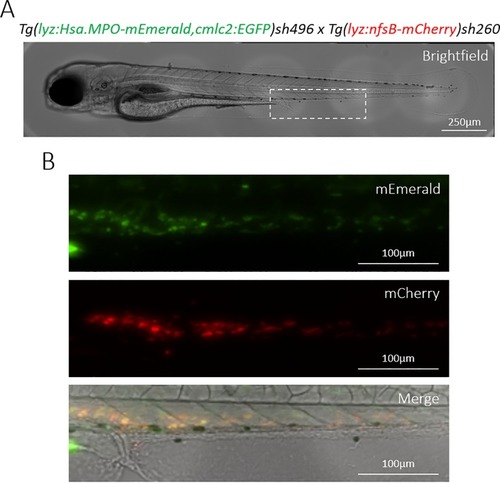
Transgenic Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496 zebrafish stably express the transgene in zebrafish neutrophils. A) A brightfield view of a double-transgenic Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; Tg(lyz:nfsB-mCherry)sh260 zebrafish larva at 3dpf. The dashed white box indicates the enlarged region shown in B). B) mEmerald and mCherry expression in the CHT of the larva shown in A). |
|
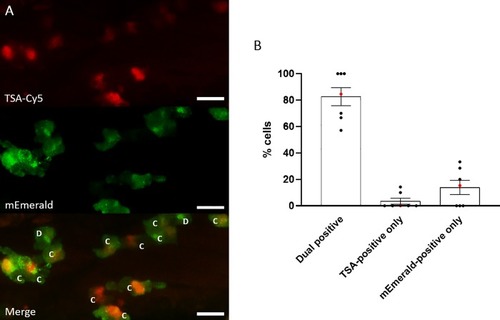
Most MPO-mEmerald-positive neutrophils are TSA-positive. A) Confocal photomicrographs shown as maximum intensity projections of lyz:MPO-mEmerald larvae fixed at 3 dpf and chemically stained with TSA-Cy5. Dual-positive (C)and mEmerald-positive only (D) cells are indicated; scale bar 10μm. B) Quantification of dual positive, TSA-positive only and mEmerald-positive only cells observed in lyz:MPO-mEmerald larvae fixed at 3 dpf and chemically stained with TSA-Cy5. Red points indicate larva shown in A). |
|
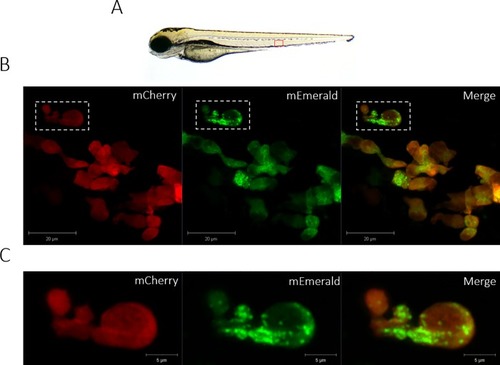
The lyz:MPO-mEmerald transgene labels intracellular neutrophil granules. A) 3dpf zebrafish larva, the field of view shown in B) is outlined by the red box. B) An Airyscanner confocal image of neutrophils within the CHT of a double-transgenic Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; Tg(lyz:nfsB-mCherry)sh260 larva at 3dpf. C) An enlarged image of the neutrophil highlighted by the dashed white box in B). Scale bars B) 20μm and C) 5μm. |
|
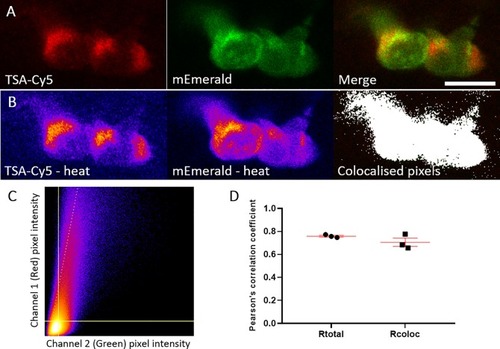
mEmerald signal colocalises with TSA signal. A) Representative photomicrograph shown as single focal plane of lyz:MPO-mEmerald (Green) larvae fixed at 3 dpf and chemically stained with TSA-Cy5 (Red). Scale bar 10 μm. B) Pseudocoloured (heat) images of Red and Green channels and image of colocalised pixels above threshold. C) Scatter plot of channel 1 (Red) vs. channel 2 (Green) of the image shown in A and B. The regression line is plotted along with the threshold level for channel 1 (vertical line) and channel 2 (horizontal line). D) Pearson's correlation coefficient for the entire image (Rtotal) or for the pixels above thresholds (Rcoloc) of 3 tested field of views. Mean +/- SEM are indicated in red. |
|
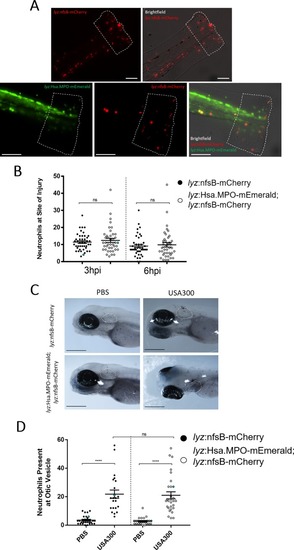
Transgene expression does not disrupt neutrophil recruitment to sites of injury or infection. A) Non-humanised (lyz:nfsB-mCherry only) and humanised (lyz:MPO-mEmerald; lyz:nfsB-mCherry) 3dpf larvae with tailfins transected to induce neutrophil recruitment; dashed outline represents the area in which neutrophils were counted. Scale bar = 250μm. B) Neutrophils present at the site of injury at 3 and 6 hours post injury (hpi); blue points denote the representative images in A). Error bars shown are mean ± SEM (n = 45 over three independent experiments); groups were analysed using an ordinary two-way ANOVA and adjusted using Bonferroni’s multiple comparisons test; ns, p>0.9999. C) Non-humanised and humanised larvae injected with either a PBS vehicle control or 1,400cfu S. aureus USA300 into the otic vesicle at 3dpf, then fixed in paraformaldehyde at 4 hours post infection (hpi) and stained with Sudan Black B to detect neutrophils; dashed white outline indicates the otic vesicle. D) Neutrophils present at the otic vesicle at 4hpi. Scale bars = 250μm. Error bars shown are mean ± SEM (n = 25 over two independent experiments); groups were analysed using an ordinary two-way ANOVA and adjusted using Bonferroni’s multiple comparisons test. ****, p<0.0001; ns, p>0.9999. |
|
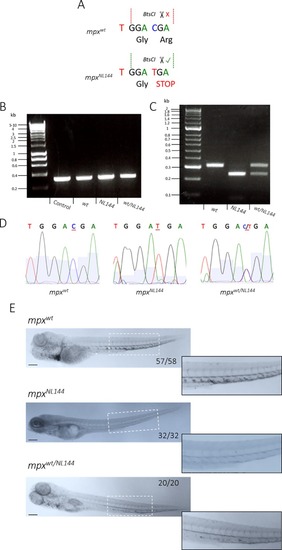
Genotyping and verifying mpx-null zebrafish larvae. A) Diagram of a WT (mpxwt) and mutated (mpxNL144) gene, showing the BtsCI restriction site cutting only the mutated mpxNL144 gene. B) PCR amplification of the mpxgene from the genomic DNA of mpxwt, mpxwt/NL144 and mpxNL144 fish–fragment 312bp; control DNA is a positive control from a separate genotyping experiment. Hyperladder 1kb. C) Diagnostic digest of the PCR product from mpxwt, mpxwt/NL144 and mpxNL144fish. Band sizes: mpxwt- 312bp, mpxNL144- 230bp, mpxwt/NL144- 312bp and 230bp. Hyperladder 100bp plus. D) DNA sequencing of the PCR products to confirm the accuracy of the BtsCI digest. E) mpxwt, mpxNL144 and mpxwt/NL144 larvae fixed at 4dpf and stained with Sudan Black B. Larvae with at least one functional mpx allele stained (57/58 mpxwt, 20/20 mpxwt/NL144) and larvae that do not produce Mpx did not stain (32/32 mpxNL144). Inset shows an enlarged view of the region indicated by the dashed white box. Scale bar = 200μm. |
|
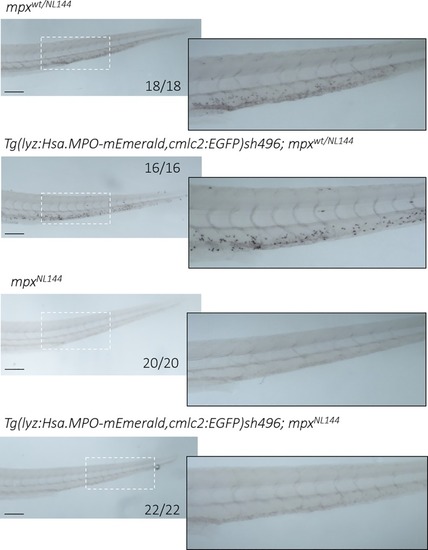
Larvae expressing only human MPO do not stain with myeloperoxidase-dependent Sudan Black B. Four groups of larvae were fixed at 4dpf and stained with Sudan Black B: mpxwt/NL144, Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxwt/NL144, mpxNL144 and Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxNL144. mpxwt/NL144 and Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxwt/NL144 stained (18/18, 16/16 respectively); mpxNL144 and Tg(lyz:Hsa.MPO-mEmerald,cmlc2:EGFP)sh496; mpxNL144 did not stain (20/20, 22/22 respectively). Dashed outline indicates the enlarged region shown adjacent. Scale bar = 200μm. |