- Title
-
Amiodarone Induces Overexpression of Similar to Versican b to Repress the EGFR/Gsk3b/Snail Signaling Axis during Cardiac Valve Formation of Zebrafish Embryos
- Authors
- Lee, H.C., Lo, H.C., Lo, D.M., Su, M.Y., Hu, J.R., Wu, C.C., Chang, S.N., Dai, M.S., Tsai, C.T., Tsai, H.J.
- Source
- Full text @ PLoS One
|
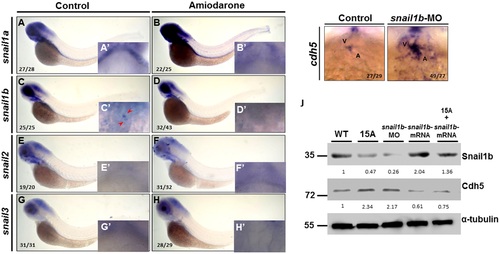
Amiodarone causes the ectopic expression of cdh5 through its repression of snail1b expression. (A-H) WISH of snail1a (A, B) snail1b (C, D), snail2 (E, F) and snail3 (G, H) in indicated embryos during 72 hpf. Control embryos were treated with DMSO. 72 hpf zebrafish embryos treated with 15 µM Amiodarone from 55 to 72 hpf. (A-H) Lateral view of 72 hpf embryos. (A′ and H′) Ventral view of 72 hpf embryos which focus on the heart region. WISH revealed that snail1a, snail2 and snail3 were not detected at the AVC, while snail1b is detected at the AVC (C′ red arrow). Incubation of 15 µM Amiodarone from 55 to 72 hpf did not influence snail1a or snail1b at head region, but the expression of snail1b at AVC was lost (D′ red arrow). (I) WISH of cdh5 in control-MO or snail1b-MO-injected embryos. (J) Western blot analysis of Cdh5 protein level in control-MO (Ctrl), Amiodarone-treated embryos (15A), snail1b-MO-injected embryos, snail1b-mRNA-injected embryos, and Amiodarone-treated plus snail1b-mRNA-injected embryos. V: ventricle, A: atrium. The number of embryos displaying similar pattern was indicated in each figure. The relative intensities of each protein were as indicated. The α-tubulin was used as an internal control. EXPRESSION / LABELING:
PHENOTYPE:
|
|
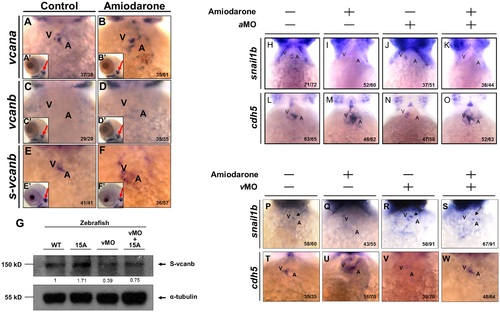
Reduced Snail1b and increased Cdh5 caused by Amiodarone treatment during valve formation are dependent on S-vcanb. Using WISH to detect the expression of vcana (A, B), vcanb (C, D) and s-vcanb (E, F) in embryos at 72 hpf was indicated. The head region of embryos treated with either DMSO (Control; A, C, E) or 15 µM Amiodarone (B, D, F) from 55 to 72 hpf. (A′-F′) indicates the head region of each embryo. Red arrows indicate the otic vesicle. The vcana and s-vcanb in the Amiodarone-treated embryos were highlighted. Their ectopic expression occurred only at the heart region. In other tissues, such as otic vesicle, no change was observed. (G) Western blot analysis of S-vcanb protein in wild-type embryos, embryos at 72 hpf after treatment with 15 µM Amiodarone from 55 to 72 hpf (15 A), embryos injected with s-vcanb-MO at one-cell stage (lane 3), and embryos injected with s-vcanb-MO at one-cell stage combined with 15 µM Amiodarone treatment from 55 to 72 hpf (lane 4). The α-tubulin gene served as the internal control. (H-W) Using WISH to detect the expressions of snail1b (H-K, P-S) and cdh5 (L-O, T-W) in WT embryos (H, L, P, T), Amiodarone-treated embryos (I, M, Q, U), vcana-MO-injected embryos (J, N), s-vcanb-MO-injected embryos (R, V), Amiodarone-treatment combined with vcana-MO injection (K, O), and Amiodarone-treatment combined with s-vcanb-MO injection (S, W). V: ventricle, A: atrium. The number of embryos displaying a similar pattern was indicated in each figure. EXPRESSION / LABELING:
PHENOTYPE:
|
|
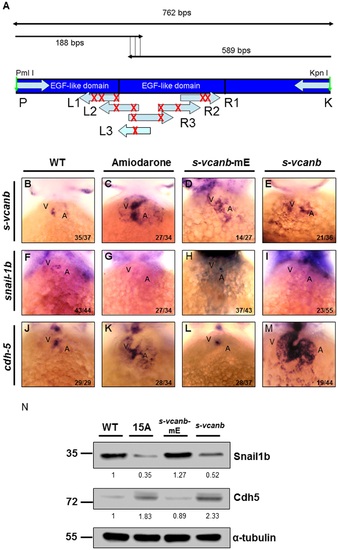
Amiodarone influences S-vcanb/Snail1b/Cdh5 signaling pathway through the EGF motif of S-vcanb. (A) Diagram illustrating the mutation sites of S-vcanb at the EGF motif. Six primers (L1–3 and R1–3) containing eight mutation sites (G′C, indicated with X), which abolish the relativity of EGF motif, were used to generate the pS-vcanb-mE. WISH of s-vcanb (B-E), snail1b (F-I) and cdh5 (J-M) in embryos treated with Amiodarone (C, G, K), overexpressed mutation form of s-vcanb-mE (D, H, L), or wild-type s-vcanb (E, I, M). In Amiodarone-treated embryos, s-vcanb (C) and cdh5 (K) were ectopically expressed, but snail1b (G) was lost. In embryos overexpressed in the mutated form of s-vcanb-mE, s-vcanb (D) was increased, but snail1b (H) and cdh5 (L) levels were similar to those of control group (F and J). In embryos overexpressed with the wild-type form of s-vcanb, s-vcanb was increased, but snail1b was lost, while cdh5 was greatly ectopically expressed. (N) Western blot analysis of Snail1b and Cdh5 in wild-type embryos, 72 hpf zebrafish embryos treated with 15 µM Amiodarone from 55 to 72 hpf (15 A), overexpressed mutation form of s-vcanb-mE, and wild-type s-vcanb embryos. Overexpressive wild-type s-vcanb embryos displayed reduced Snail1b and increased Cdh5 patterns. The number of embryos displaying a similar pattern was indicated in each figure. The relative intensities of each protein were as indicated. The α-tubulin was used as an internal control. EXPRESSION / LABELING:
PHENOTYPE:
|
|
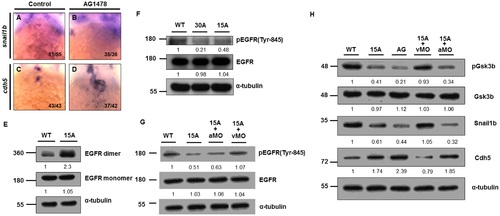
Amiodarone-induced zebrafish s-vcanb can repress EGFR signaling. WISH of snail1b (A, B) and cdh5 (C, D) in control (A, C) and AG1478 (B, D)-treated embryos. When AG1478 inhibited EGFR activity, snail1b (B) in the AVC was lost, but cdh5 (D) was greatly induced. The number of embryos displaying a similar pattern was indicated. (E) EGFR-targeting antibodies were used to depict EGFR monomers and dimers in 72 hpf WT and Amiodarone-treated embryos. (F) Amiodarone can repress the phosphorylation of EGFR on Tyr-845. (G) Knockdown of s-vcanb (vMO) can rescue the reduction of EGFR phosphorylation on Tyr-845 in Amiodarone-treated embryos, but knockdown of vcana (aMO) cannot. (H) Although Gsk3b activity was increased in Amiodarone-treated embryos, it could be rescued by knockdown of s-vcanb, but not vcana. 15A: 15µM Amiodarone treatment; 30A: 30 µM Amiodarone treatment. The relative intensities of each protein were as indicated. The α-tubulin was used as an internal control. EXPRESSION / LABELING:
PHENOTYPE:
|
|
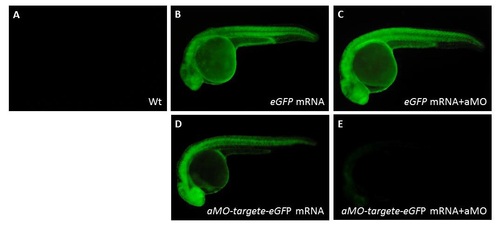
Validating specific inhibition of vcana-MO used in zebrafish embryos. The vcana-MO (12 ng; aMO) combined with its counterpart mRNA (50 pg) was injected into one-celled stage of zebrafish embryos. Uninjected embryos (A), embryos injected with eGFP mRNA alone (B), and embryos injected with eGFP mRNA plus vcana-MO (C), which served as the control group. The aMO-target-eGFP fusion protein was detected at 24 hpf in embryos injected with a aMO-target-eGFP mRNA (D). The aMO-target-eGFP fusion protein was nearly undetectable at 24 hpf in embryos injected with aMO-target-eGFP mRNA plus vcana-MO (E). |