- Title
-
Notch signalling is required for the formation of structurally stable muscle fibres in zebrafish
- Authors
- Pascoal, S., Esteves de Lima, J., Leslie, J.D., Hughes, S.M., and Saúde, L.
- Source
- Full text @ PLoS One
|
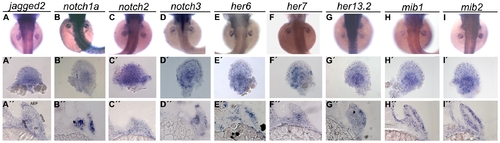
Expression pattern of Notch signalling pathway genes during pectoral fin development. Whole-mount in situ hybridization of 48 hpf embryos shows strong expression of jagged2 (n = 20) (A), notch1a (n = 22) (B), notch2 (n = 22) (C), notch3 (n = 20) (D), her6 (n = 25) (E), her7 (n = 23) (F), her13.2 (n = 23) (G), mib1 (n = 22) (H) and mib2 (n = 25) (I) in the pectoral fins. At this time point it is possible to detect expression of jagged2 (A′, A′′) and notch3 (D′, D′′) lining the base of the apical ectodermal fold and in the myogenic mesenchyme, notch1a (B′, B′′), her6 (L′, L′′), her13.2 (N′, N′′), mib1 (H′, H′′) and mib2 (I′, I′′) in the myogenic mesenchyme and notch2 (J′, J′′) and her7 (M′, M′′) in the entire fin. (A′–I′) Detached pectoral fins with distal to the top. (A′′-I′′) Transversal sections at the level of pectoral fins. AEF, apical ectodermal fold; dmm, dorsal myogenic mesenchyme; vmm, ventral myogenic mesenchyme; cc, chondrogenic condensation. EXPRESSION / LABELING:
|
|
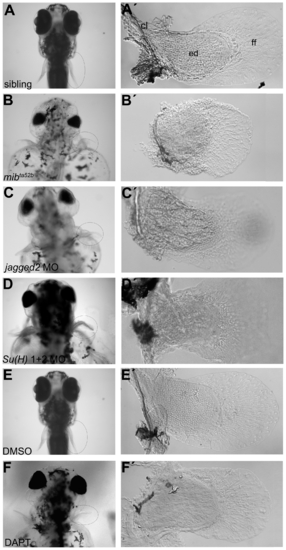
Abnormal pectoral fins are formed in Notch signalling disrupted larvae. (A-F) Live pictures of 5 dpf larvae. (A′) A pectoral fin from a sibling embryo showing the cartilaginous endoskeletal disc with individualized cells surrounded by thin matrix deposits, the fin fold and the chleitrum (n = 17) (E, E′). A similar pectoral fin was found in a DMSO-treated embryo (n = 9). Pectoral fins of Notch signalling disrupted embryos such as mibta52b (n = 18) (B, B′), jagged2 (n = 10) (C, C′) and Su(H)1+2 (n = 12) (D, D′) morphants and DAPT-treated embryos (n = 10) (F, F′) showing disorganized endoskeletal disc cells. cl, chleitrum; ed, endoskeletal disc; ff, fin fold. PHENOTYPE:
|
|
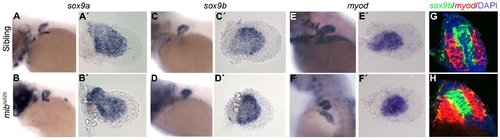
Cartilage and muscle masses are specified in mibta52b mutants. Whole-mount in situ hybridization of 68 hpf siblings (n = 15) (A, A′), (n = 12) (C, C′), (n = 14) (E, E′) and mibta52b mutants (n = 12) (B, B′), (n = 15) (D, D′), (n = 11) (F, F′) using sox9a and sox9b as cartilage markers and myod as a muscle marker, showing a slight upregulation of these genes in mibta52b mutant pectoral fin. (A-F) Whole larvae. (A′-F′) Detached pectoral fins with distal to the right. Double fluorescent in situ hybridization on sections using sox9b (green) and myod (red), counterstained with DAPI to label the nuclei (blue) shows no signs of cell mixing in a sibling (n = 7) (G) and a mibta52b mutant pectoral fins (n = 9) (H). An upregulation of these genes is observed in mibta52b mutant pectoral fins when compared with the sibling. |
|
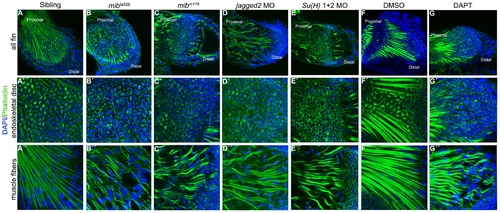
Notch signalling is crucial for skeletal muscle integrity and stress fibres formation. Immunostaining of 5 dpf pectoral fins (A–G′′) with DAPI and phalloidin to label nuclei (blue) and filamentous actin (green), respectively. Stress fibres in the endoskeletal disc cells and intact skeletal muscle fibres are formed in the pectoral fins of siblings (n = 20) (A–A′′) and in DMSO-treated embryos (n = 17) (F-F′′). In the pectoral fins were Notch signalling was disrupted like in mibta52b (n = 22) (B–B′′) and mibm178 (n = 10) (C–C′′) mutants, jagged2 (n = 11) (D–D′′) and Su(H)1+2 (n = 10) (E–E′′) morphants and in DAPT-treated embryos (n = 16) (G–G′′) the endoskeletal disc cells present high levels of actin at the periphery and the skeletal muscle fibres are wavy with gaps between them. PHENOTYPE:
|
|
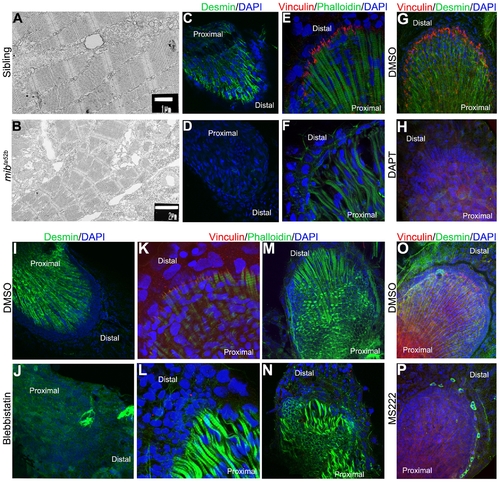
Mechanical weak muscles fibres are produced when Notch signalling is perturbed. Transmission electronic microscopy was performed in 5 dpf pectoral fins of siblings (A) and mibta52b mutants (B) to analyse the ultrastructure of skeletal muscle fibres. The myofibrils with clear aligned sarcomeres are formed in the sibling embryo (A), while disintegrating myofibrils with poorly aligned sarcomeres are found in mibta52b mutants (B). Immunostaining performed in pectoral fins at 5 dpf using Desmin, Vinculin, phalloidin and DAPI to label the Z-discs of the sarcomeres, the zone where the skeletal muscle fibres insert distally, filamentous actin and the cell nuclei, respectively (C–P) demonstrate that Desmin (n = 10) (D) and Vinculin (n = 12) (F) are downregulated in mibta52b mutants when compared with their siblings (n = 10) (C, E). The same is observed in DAPT-treated embryos at 48 hpf and fixed at 5 dpf (n = 9) (H), when compared with the DMSO-treated embryos (n = 10) (G). In embryos treated with blebbistatin, Desmin (n = 9) and Vinculin (n = 12) are also downregulated (J, L) when compared with the DMSO-treated embryos (n = 12) (I, K). In blebbistatin-treated embryos, the endoskeletal disc cells present high levels of actin at the periphery and the skeletal muscle fibres are wavy with gaps between them (n = 15) (N). A down-regulation of Desmin and Vinculin is also observed in MS222-treated embryos (n = 14) (P) when compared with the control embryos (n = 13) (O). |
|
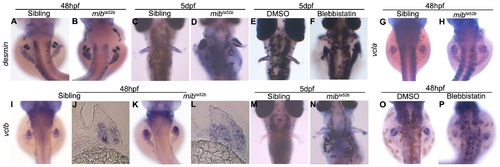
desmin and vinculin are not transcriptionally downregulated in mibta52b mutants. Whole-mount in situ hybridization using desmin, vcla and vclb as mRNA probes. At 48 hpf, desmin is expressed at similar levels in the myogenic mesenchyme of the pectoral fins of siblings (n = 18) (A) and mibta52b mutants (n = 20) (B). At 5 dpf, the expression of desmin becomes stronger in mibta52b mutants (n = 19) (D) when compared with the sibling (n = 16) (C). Blebbistatin-treated embryos (n = 12) (F) show a similar level of desmin expression as control DMSO-treated (n = 10) (E). vcla is expressed strongly in the somites and has a faint expression in the sibling pectoral fins (n = 15) (G), the same expression pattern can be observed in the mibta52b mutant (n = 18) (H). vclb is expressed in the myogenic mesenchyme at 48 hpf in both siblings (n = 18) (I), (n = 5) (J) and mibta52b mutants (n = 19) (K), (n = 5) (L). At 5 dpf, the expression of vclb is poorly detected in the sibling embryos (n = 15) (M) and is still detected in mibta52b mutants (n = 16) (N). Transversal sections at the level of the pectoral fin of 48 hpf embryos are shown (J, L). Blebbistatin-treated embryos (n = 10) (P) show a similar level of vclb expression as control DMSO-treated (n = 12) (O). |
|
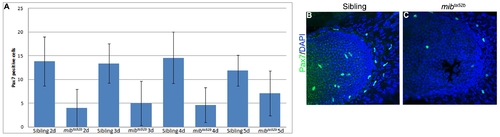
Pax7 muscle progenitor cells are depleted in mibta52b mutant pectoral fins. (A) Number of Pax7-positive cells in siblings and mibta52b mutants at time points 2 dpf (sibling n = 6, mibta52b n = 7, t-test p = 0.004), 3 dpf (sibling n = 13, mibta52b n = 10, t-test p = 0.0002), 4 dpf sibling n = 7, mibta52b n = 14, t-test p = 0.0019) and 5 dpf (sibling n = 14, mibta52b n = 15, t-test p = 0.003). Error bars = SD. 5 dpf pectoral fins immunostained for Pax7 and DAPI to label muscle progenitor cells and nuclei, respectively, in (B) siblings and (C) mibta52b mutants. |
|
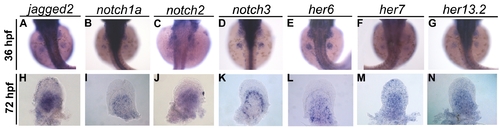
Expression pattern of Notch signalling pathway genes at early and late time points of pectoral fin development. Expression of jagged2 (n = 25) (A), notch1a (n = 27) (B), notch2 (n = 20) (C), notch3 (n = 25) (D), her6 (n = 22) (E), her7 (n = 20) (F) and her13.2 (n = 20) (G) can be detected in the entire pectoral fin at 36 hpf. Later in development at 72 hpf the expression of these genes is still detected in the detached pectoral fins with distal to the top (H–N). |
|
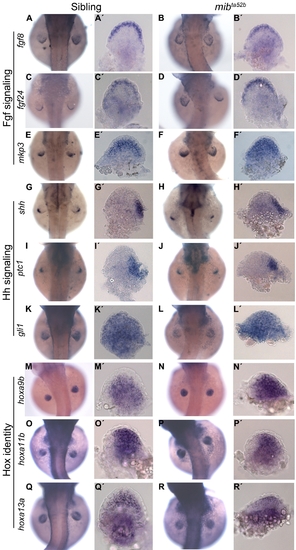
Proximal-distal and anterior-posterior patterning and Hox identity are not affected in mibta52b mutants. The Fgf signalling components fgf8 (n = 15) (A, A′) and fgf24 (n = 12) (C, C′) are expressed in the apical ectodermal fold of 48 hpf sibling embryos. The same pattern of expression is observed in the pectoral fins of mibta52b mutants (n = 12) (B, B′), (n = 14) (D, D′). The Fgf signalling downstream target mkp3 is expressed in a gradient through a distal to proximal direction in fin mesenchymal cells of sibling embryos (n = 16) (E, E′). The expression of mkp3 is upregulated proximally in mibta52b mutants (n = 18) (F, F′). The Hh signalling components shh and ptc1 are expressed in the zone of polarizing activity and gli1 in the mesenchymal cells that compose the fin. No differences in the expression pattern can be observed between siblings (n = 12) (G, G′), (n = 15) (I, I′), (n = 10) (K, K′) and mibta52b mutants (n = 10) (H, H′), (n = 12) (J, J′), (n = 11) (L, L′). The expression of hoxa9b, hoxa11b and hoxa13a reveals no differences in the patterns of these genes in siblings (n = 9) (M, M′), (n = 11) (O, O′), (n = 10) (Q, Q′) and mibta52b mutants (n = 10) (N, N′), (n = 9) (P, P′), (n = 11) (R, R′). (A–R) Whole embryos. (A′–R′) Detached pectoral fins with distal to the top and posterior to the right. |