- Title
-
Kinocilia mediate mechanosensitivity in developing zebrafish hair cells
- Authors
- Kindt, K.S., Finch, G., and Nicolson, T.
- Source
- Full text @ Dev. Cell
|
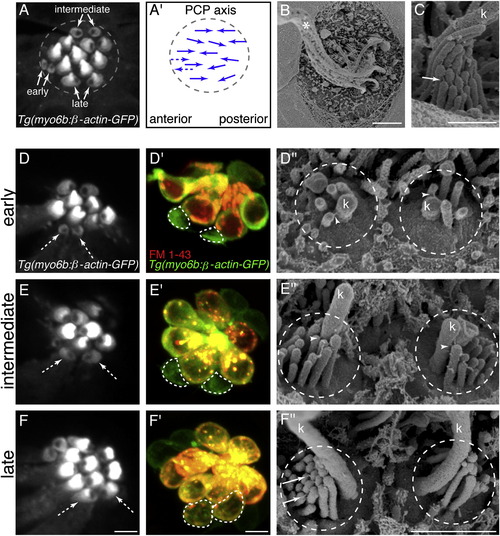
Direct Comparison of Hair-Bundle Development Using Transgenic β-actin-GFP, FM 1-43, and SEM(A) Top-down view of neuromast hair bundles labeled with Tg(myo6b:β-actin-GFP). Three stages of immature hair-bundle development can be observed: early, intermediate, and late. The unlabeled region on either the caudal or rostral side of each bundle is the insertion point of the kinocilium.(A2) Arrows indicate planar polarity and functional polarity of hair bundles in (A). Dashed arrows indicate predicted planar polarity. PCP, planar cell polarity.(B) SEM image of a neuromast at 3 dpf; asterisk denotes the many tall kinocilia (k).(C) SEM image of a mature hair bundle.(D, E, and F) Confocal images of hair bundles expressing β-actin-GFP. White dashed arrows indicate hair bundles of respective stage examined.(D′, E′, and F′) White dashed lines outline cell bodies after FM 1-43 label of hair bundle examined in (D), (E), and (F).(D′′, E′′, and F′′) SEM images of hair bundles in (D), (E), and (F). All images were taken at 3 dpf. White arrowheads denote kinocilial links; solid white arrows denote tip links. Anterior is left; dorsal is up. Scale bars, 2.5 μm (A, B, D, E and F), 500 nm (C), 5 μm (D′, E′, and F′), and 1 μm (D′′, E′′, and F′′). See also Figure S1. |
|
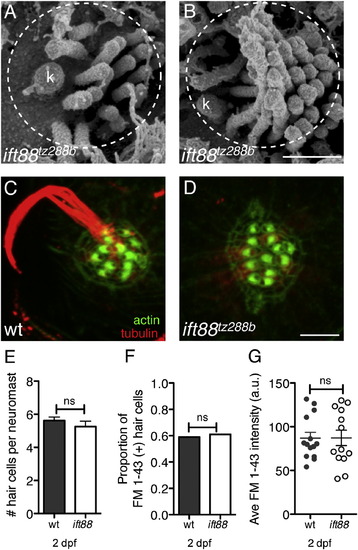
ovl/ift88 Mutant Hair Cells Lack Kinocilia yet Develop Normally(A and B) SEM image of an ift88tz288b immature (A) and mature (B) hair bundle.(C and D) Tubulin and actin stain of bundles at 5 dpf in wild-type (WT) (C) and ift88tz288b (D).(E) The average number of hair cells per neuromast at 2 dpf in wild-type and ift88tz288b neuromasts (n > 13 neuromasts). ns, not significant.(F) Proportion of FM 1-43-labeled hair cells in ift88tz288b and wild-type neuromasts at 2 dpf (n > 13 neuromasts).(G) The average FM 1-43 intensity per neuromast at 2 dpf (n > 13 neuromasts). a.u., arbitrary units.An unpaired Student’s t test was used to compare number of hair cells per neuromast in (E) and (G). A chi-square Fisher’s exact test was used to compare proportion of FM 1-43-labeled hair cells in (F). Scale bars, 500 nm (A and B) and 5 μm (C and D). Error bars are SEM. |
|
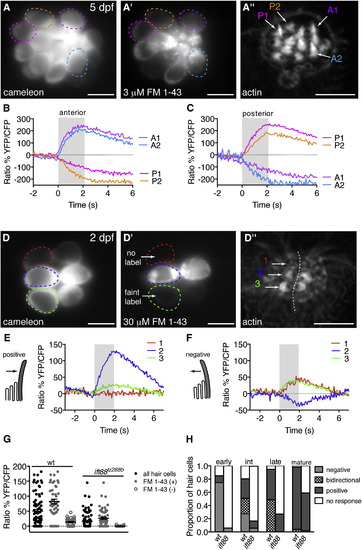
Functional Polarity of Mature and Immature Zebrafish Hair Cells(A) A 5 dpf neuromast expressing cameleon. Circles represent cells analyzed and are color coded and correspond to data in (A′)–(C).(A′ and A′′) FM 1-43 and phalloidin stain of the neuromast in (A). Two anterior-polarized hair bundles (A1 and A2) and two posterior-polarized hair bundles (P1 and P2) are indicated.(B and C) Response of four individual hair cells highlighted in (A) to the same anterior (B) and posterior (C) stimulus.(D–D′′) Same layout as (A)–(A′′) except analysis is at 2 dpf. Hair cell stages are as follows: cell 1, early; cell 2, mature; cell 3, intermediate. (D) A neuromast expressing cameleon. (D′) FM 1-43 strongly labeled a pair of mature cells (cell 2 and sister), faintly labeled a pair of intermediate cells (cell 3 and sister), and yet did not label a pair of early hair cells (cell 1 and sister). (D′′) Phalloidin label of the neuromast in (D)–(D′).(E and F) Mechanically evoked calcium responses corresponding to cells 1–3. Negative and positive deflections are indicated with schematics. Kinocilium is denoted as the tall, gray structure. Cell 1 responded to only negative deflections. Cell 2 responded to positive deflections. Cell 3 responded to both negative and positive deflections.(G) Hair-cell responses (black circles) from wild-type (n = 13 neuromasts) and ift88tz288b (n = 21 neuromasts) at 2 dpf. These same data were broken down into FM 1-43-labeled hair cells (gray circles) and FM 1-43-unlabeled hair cells (open gray circles).(H) Proportion of negative, bidirectional, and positive responses at 2 dpf (n = 188 wild-type and 72 ift88tz288b cells).Errors bars are SEM. Scale bars, 5 µm. For stimulus controls, see also Figure S2. |
|
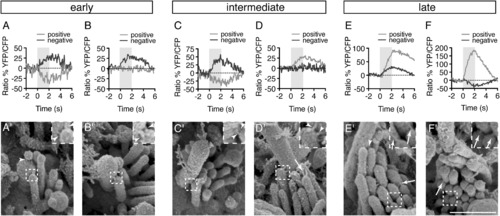
Correlative Calcium Imaging and SEM Reveal a Change in Link Architecture during Development(A–F) Calcium responses were measured in response to positive and negative hair-bundle deflections from cells at each stage of bundle development.(A′–F′) SEM images of the hair bundles from hair cells represented in (A)–(F).All images were taken at 3 dpf. Arrowheads indicate kinocilial links; arrows indicate tip links. Scale bar, 500 nm. Inset is 2× magnification. For additional link examples see also Figure S3. |
|
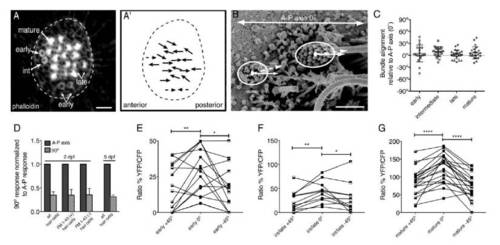
Related to Figure 1. The morphological and functional polarity of neuromast hair bundles is tuned to the A-P axis. |
|
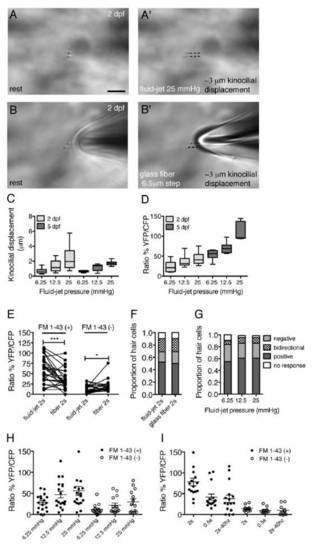
Related to Figure 3. Quantification of the fluid-jet stimulus at 2 dpf and 5 dpf. |
|
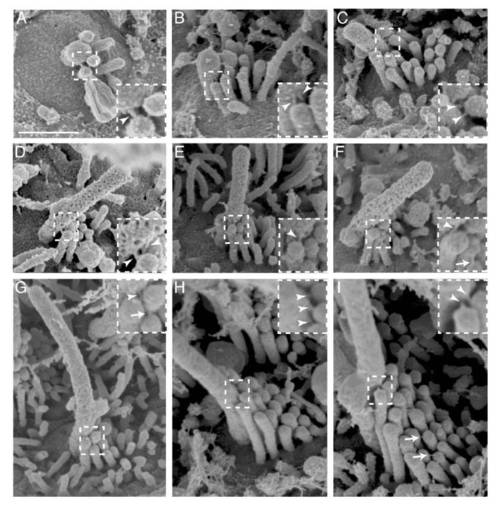
Related to Figure 5. Kinocilial links are present at all stages of hair bundle development. (A-I) SEM images of early (A and B), intermediate (C, D, E and F), late (G and H) late and mature (I) hair bundles. Arrowheads indicate kinocilial links, while arrows indicate tip links. Scale bar is 500 nm, inset is 2X magnification |
|
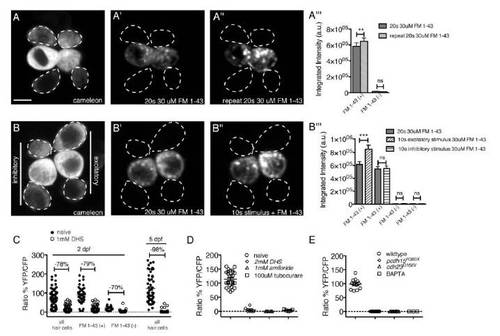
Related to Figure 6. FM 1-43 does not label all mechanosensitive hair cells at 2 dpf. |
Reprinted from Developmental Cell, 23(2), Kindt, K.S., Finch, G., and Nicolson, T., Kinocilia mediate mechanosensitivity in developing zebrafish hair cells, 329-341, Copyright (2012) with permission from Elsevier. Full text @ Dev. Cell