- Title
-
A model of Costeff Syndrome reveals metabolic and protective functions of mitochondrial OPA3
- Authors
- Pei, W., Kratz, L.E., Bernardini, I., Sood, R., Yokogawa, T., Dorward, H., Ciccone, C., Kelley, R.I., Anikster, Y., Burgess, H.A., Huizing, M., and Feldman, B.
- Source
- Full text @ Development
|
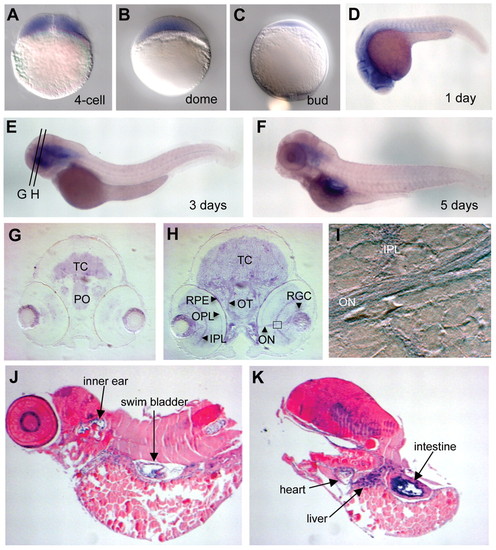
Zebrafish opa3 expression in the brain, eye and other organs during embryogenesis. (A-F) Whole-mount in situ stains of opa3 mRNA. Embryonic stages are as labeled. Orientations: A-F,J-K, lateral views; G-I, frontal views. (G-I) opa3 expression in brain and eyes. (G,H) Transverse sections (approximate positions shown in E) through a 3-day-old opa3-stained embryo. TC, tectum; PO, pre-optic region; RPE, retinal pigment epithelium; OPL, outer plexiform layer; IPL, inner plexiform layer; OT, optic tract; ON, optic nerve; RGC, retinal ganglion cells. I shows a higher magnification view of the boxed region in H. (J,K) opa3 mRNA expression in a 5-day-old embryo. Embryos were laterally sectioned and counterstained with nuclear Fast Red. |
|
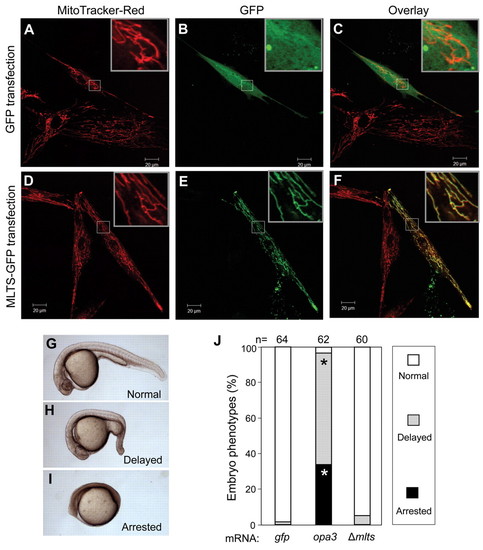
Zebrafish Opa3 has functional mitochondrial leader and targeting sequences (MLTS). (A-F) Targeting assay. Human fibroblasts were transfected with plasmids expressing GFP or GFP linked to the MLTS of Opa3 (MLTS-GFP; Opa3 residues A1-S34 fused to the N terminus of GFP). A and D show the MitoTracker Red stains alone; B and E show GFP fluorescence; and C and F show the overlap of both signals. An enlarged inset in each image allows better visualization. (G-I) Gain-of-function assay. Wild-type embryos were injected with 200 pg of opa3 mRNA or mutated opa3 mRNA (Δmlts) from an opa3 vector in which the MLTS was deleted in frame (residues M1-K28 deleted, A29 converted to M). Embryonic phenotypes were scored at day 1. (J) Incidence of overexpression phenotypes. The number of embryos scored for each mRNA injection is indicated. Asterisks (*) indicate statistically significant increases (chi-square test, P<10–6) between the opa3 mRNA injected embryos and Δmlts mRNA-injected embryos. No difference was seen between Δmlts mRNA-injected embryos and gfp mRNA-injected embryos (P=0.2789). |
|
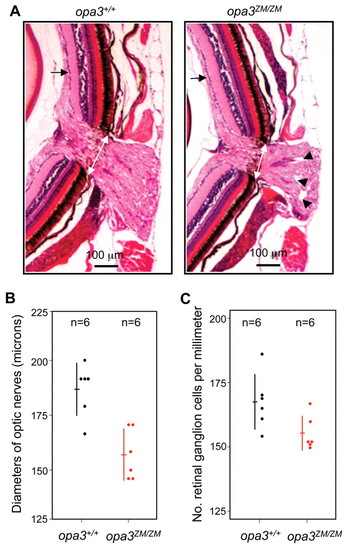
Optic nerve deficits in opa3ZM/ZM mutants. (A) Sections of opa3+/+ and opa3ZM/ZM adult retinas. Three 1-year-old adult fish for each genotype were coronally sectioned and Hematoxylin and Eosin stained. One representative section is shown for each genotype. White arrows indicate optic nerve diameters and measurement points. Arrowheads indicate optic nerve lesions. Black arrows indicate retinal ganglion cells. (B,C) Comparison of optic nerve diameters (B) and retinal ganglion cell numbers (C) in opa3+/+ and opa3ZM/ZM adult fish. Each dot represents a single measurement, horizontal lines show the average and vertical lines show the standard deviation. Red lines and data points are significantly different from black lines and data points (t-test, P<0.015). PHENOTYPE:
|
|
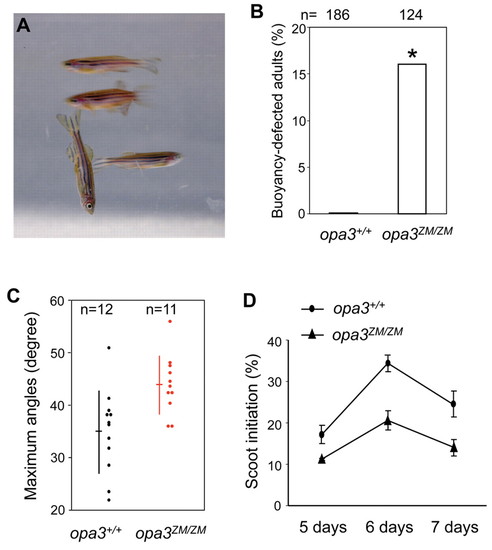
Behavioral anomalies of opa3ZM/ZM mutants. (A) Image of one abnormally swimming opa3ZM/ZM adult fish and three normal opa3ZM/ZM siblings. (B) Increased buoyancy in opa3ZM/ZM adult fish. Asterisk (*) indicates the statistical significance of the increase (chi-square test, P=2.1e–7). (C) Exaggerated vertical turns by opa3ZM/ZM adult fish. Each dot represents combined average of maximum ascent and descent angles observed for an individual fish. Horizontal lines show the overall average and vertical lines show the standard deviations. Red lines and data points are significantly different from black lines and data points (t-test, P=0.0055). (D) Reduced spontaneous scoot initiation by opa3ZM/ZM larvae. Circles show the average scoot frequency for four groups of 25 opa3+/+ larvae and triangles show the average scoot frequency for four groups of 25 opa3ZM/ZM larvae, each measured on three consecutive days. A significant reduction was observed in opa3ZM/ZM larvae (ANOVA test, F[1,9]=23.4, P=0.001). |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
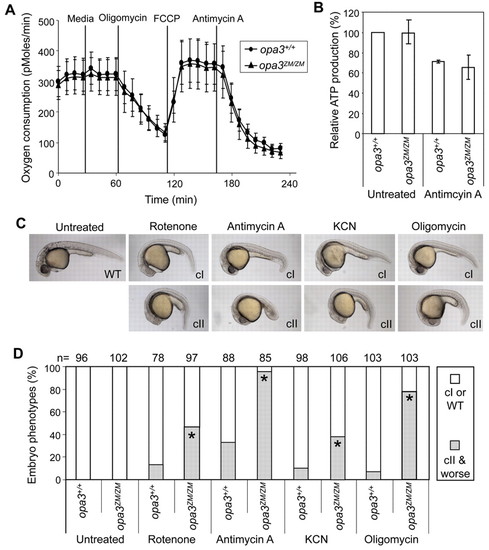
Loss of Opa3 does not affect overall energy metabolism, yet sensitizes embryos to inhibitors of the electron transport chain. (A) Equivalent mitochondrial respiration in opa3+/+ and opa3ZM/ZM embryos. Mitochondrial activity was inferred from the rate of oxygen depletion, as periodically measured in the micro-environment adjacent to groups of four 14-somite stage embryos, before and after the addition of compounds selected to maximize (FCCP) or minimize (oligomycin, antimycin A) the rate of oxidative phosphorylation. Short vertical lines indicate standard deviations and tall vertical lines show the times of each drug injection. (B) Equivalent ATP production in opa3+/+ and opa3ZM/ZM embryos. opa3+/+ embryos and opa3ZM/ZM embryos were either mock treated or treated with 1 ng/ml antimycin A starting at the four-cell stage and harvested on day 1 for ATP measurements. (C) Effects of ETC inhibitors on embryonic development. Embryos were treated with four different ETC inhibitors and scored at day 1. Resulting phenotypes are shown and categorized as class I (cI) and class II (cII). (D) Sensitivity of opa3ZM/ZM embryos to ETC inhibitors. A substantially larger fraction of opa3ZM/ZM embryos displayed class II phenotypes after drug treatment. Asterisks (*) indicate the statistical significance of the increases (chi square test, P<0.0001) in comparisons with the controls on the left. PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|