- Title
-
Shp2 Knockdown and Noonan/LEOPARD Mutant Shp2-Induced Gastrulation Defects
- Authors
- Jopling, C., van Geemen, D., and den Hertog, J.
- Source
- Full text @ PLoS Genet.
|
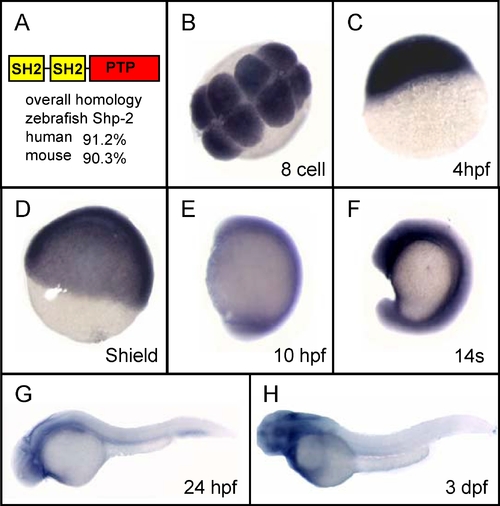
Zebrafish Shp2 Is Conserved and Is Expressed Ubiquitously during Development EXPRESSION / LABELING:
|
|
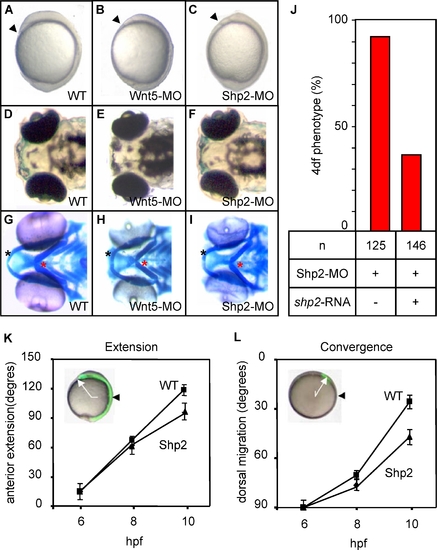
Shp2-MO–Induced CE Cell Movement Defects |
|
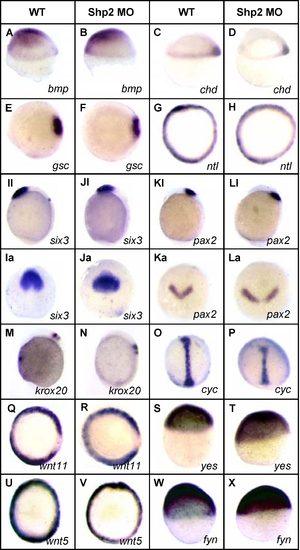
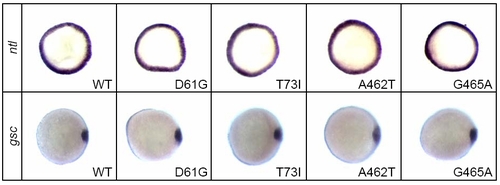
Shp2 Knockdown Did Not Affect Cell Specification |
|
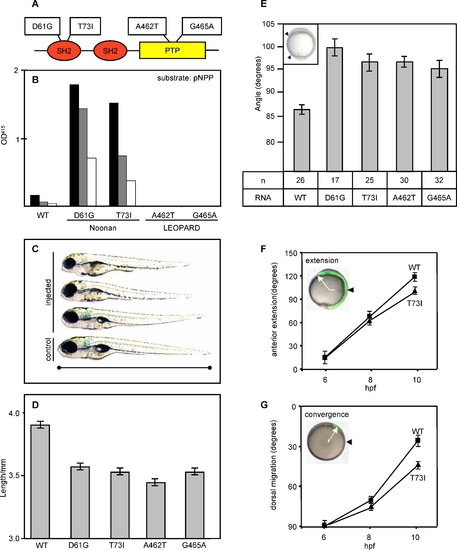
NS and LS Mutant Shp2 Expression Induced CE Cell Movement Defects during Gastrulation |
|
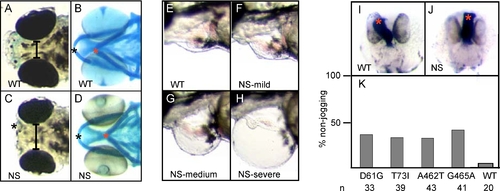
Craniofacial and Heart Defects upon NS or LS RNA Injection |
|
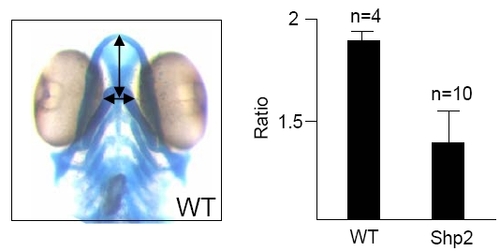
Morphometry of the Hammerhead Phenotype PHENOTYPE:
|
|
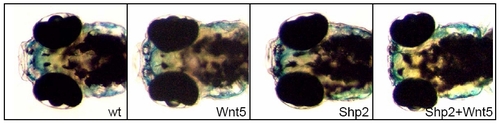
Shp2 and Wnt5 Knockdown Acts Synergistically to Induce a Hammerhead Phenotype at 4 dpf PHENOTYPE:
|
|
Expression of NS- and LS-Shp2 Did Not Induce Defects in Cell Specification |