- Title
-
Mef2s are required for thick filament formation in nascent muscle fibres
- Authors
- Hinits, Y., and Hughes, S.M.
- Source
- Full text @ Development
|
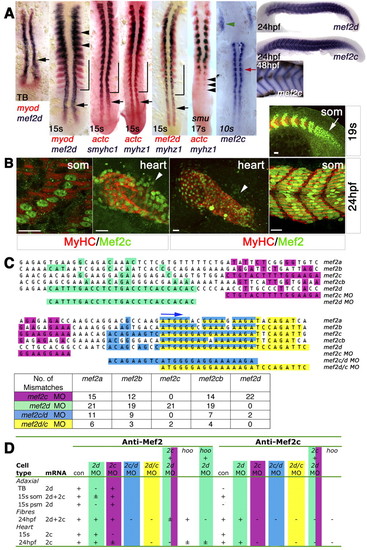
Mef2d and Mef2c expression during zebrafish skeletal myogenesis and cardiogenesis. (A) In situ mRNA hybridisation of mef2d, mef2c, myod, actc1 (actc), smyhc1 and myhz1 viewed in dorsal (anterior to top) or lateral (right-most panels; anterior to left, dorsal up) flatmount. Mef2d, myod and actc1 mRNAs coincide in early adaxial slow (black arrow) and lateral fast (black arrowheads) myoblasts and precede smyhc1 and myhz1 in differentiated slow (bracket) and fast muscle fibres. Sequential expression of actc1 and myhz1 during fast muscle differentiation is confirmed in smu (smo) mutant, which lacks slow muscle. Mef2c expression commences as adaxial cells form slow fibres (red arrow) and in bilateral heart fields (green arrowhead). Mef2c expression in fast muscle parallels terminal differentiation. TB, tailbud; s, somite. (B) Immunodetection of Mef2 and MyHC in wholemount embryos viewed in dorsal (heart; anterior to top) or lateral [somite (som); anterior to left, dorsal up] flatmount. Confocal single scans of anti-Mef2c and z-stacks of anti-Mef2 antibodies detect muscle nuclei in skeletal myoblasts in the presomitic mesoderm (arrow), muscle fibres, heart tube and undifferentiated cardiomyoblasts (arrowheads). Scale bars: 20 µm. (C) Sequence alignment of start codon region (blue arrow) of five zebrafish Mef2 genes and comparison with MOs employed. Coloured squares highlight identity with the MO. The table shows the number of mismatches. (D) Location of anti-Mef2 and anti-Mef2c immunoreactivity in relation to mef2d and mef2c mRNAs and the effect of the indicated MO combinations (see Figs S1-S3 in the supplementary material). +, ± and-indicate normal, low and no nuclear reactivity, respectively; where there is no entry, nuclear reactivity was not determined. |
|
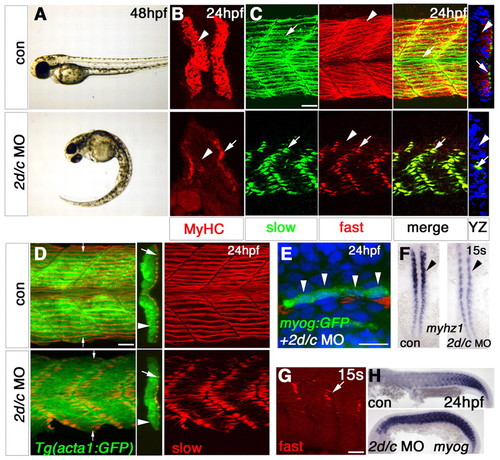
Mef2 knockdown blocks fast muscle myofibril assembly. Zebrafish embryos in bright field (A,F,H), after immunodetection of all MyHC (B), slow MyHC (C-E) or fast MyHC (C,G) or in situ mRNA hybridisation (F, dorsal flatmount; H, lateral flatmount). (A,B) mef2d/c MO causes tail curvature (A) and ablates MyHC (B) in fast (arrowhead), but not slow (arrow) fibres. (C) mef2d/c morphants retain fast MyHC in slow fibres (arrows), but have essentially no fast MyHC in fast fibres (arrowheads), as shown in the yz optical transverse reconstruction. (D) Injection of mef2d/c MO into Tg(acta1:GFP) fish disrupts slow myofibrilogenesis (red) but not actin reporter expression (green). yz reconstruction at small arrows. Large arrows, slow fibres; arrowheads, fast fibres. (E) myogenin:GFP DNA co-injected with mef2d/c MO into wild-type embryos yields elongated GFP-filled fibres (green) with three to four nuclei (DAPI-stained, blue, arrowheads) at 24 hpf, despite disorganised slow MyHC (red). (F) Dorsal flatmounts showing decrease in myhz1 in fast precursors (arrowhead) but no change in adaxial slow precursors. (G) In wild-type embryos, fast MyHC is present in slow fibres at 15 somites (arrow) but is lost by 24 hpf (arrows, upper panels in C). (H) Persistence of myogenin mRNA in mef2d/c morphants. Scale bars: 20 µm. EXPRESSION / LABELING:
|
|
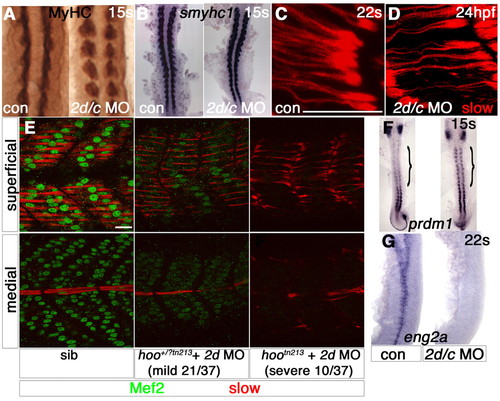
Slow muscle myofibril defects correlate with the extent of Mef2 depletion. Zebrafish embryos in bright field (A,B,F,G), after immunodetection of all MyHC (A), slow MyHC (C-E) or Mef2 (E) or in situ mRNA hybridisation (B,F, dorsal flatmounts; G, lateral flatmount). (A,B) Slow muscle differentiation at 15 somites is unaffected by mef2d/c MO (A, somites 6-10). (C,D) Immature slow fibres in mef2d/c morphant at 24 hpf (D) are of comparable maturity to nascent fibres in a 20-hpf control embryo at the same rostrocaudal position (C). (E) Injection of mef2d MO into a hootn213 heterozygote cross yielded three phenotypes at the frequencies shown. Putative heterozygotes have diminished Mef2 in both slow (superficial slice) and fast (medial slice) fibres and thinner slow myofibril bundles. Putative mutants contained no Mef2 and had immature slow fibres. (F,G) mef2d/c morphants show persistence of prdm1 in adaxial cells (F, bracket) and loss of eng2a expression (G). Scale bars: 20 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
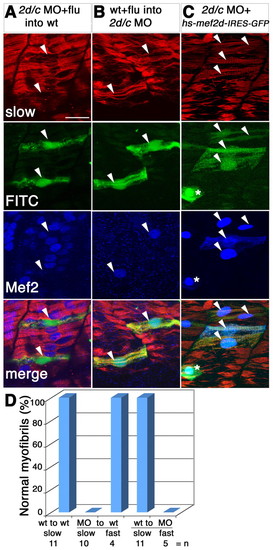
Mef2 controls slow fibre myofibril assembly cell-autonomously and rescues the morphant. Detection of slow MyHC (red), Mef2 (blue) and FITC or GFP (green) in manipulated 24-hpf zebrafish embryos. (A,B) Transplantation of FITC-dextran-labelled cells (arrowheads, green) from mef2d/c morphants into wild-type host (A) or from wild-type donor into mef2d/c morphant host (B). Note the immature character of the FITC-labelled transplanted fibres in A and the greater maturity of transplanted cells in B, compared with their neighbours. (C) Co-injection of hs-mef2d-IRES-GFP and mef2d/c MO followed by heat shock at 22 hpf rescues myofibril structure in the GFP-expressing cells, which also contain nuclear Mef2 (arrowheads). Asterisk indicates a Mef2-positive skin cell. (D) Bar chart showing quantification of transplantation results. |
|
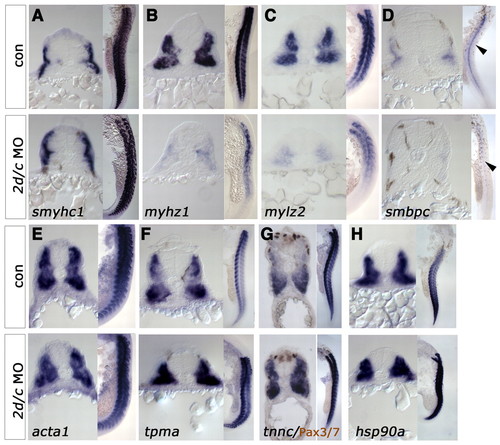
Mef2 controls a transcriptional module involved in thick filament assembly. In situ hybridisation for mRNAs encoding sarcomeric components in 24-hpf control or mef2d/c MO-injected zebrafish embryos. In each panel, cryosections of wholemount embryos, dorsal up, are to the left and flatmounts, anterior up and dorsal to right, are to the right. (A-D) Embryonic fast myhz1 (B), mylz2 (C, 22s) and smbpc (D) mRNAs are grossly downregulated in morphants. Slow fibre-specific smyhc1 mRNA (A) remains abundant. (E-G) mRNA for thin filament-related genes detected with probes to acta1 (E, 21s) or tnnc (G) are unaltered in morphants, whereas tpma (F) is upregulated. Note the lack of change in nuclear Pax3/7 protein (F, brown). (H) mRNA for the myosin chaperone protein hsp90a is also upregulated. EXPRESSION / LABELING:
|
|
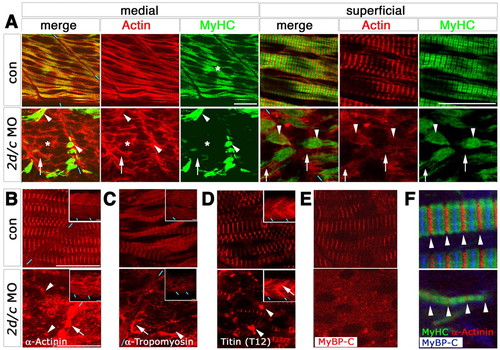
Mef2 controls a transcriptional module involved in thick filament assembly. Confocal immunofluorescence of sarcomeric components in a 24-hpf midbody somite of control or mef2d/c MO-injected zebrafish embryos in fast (A, medial) or slow (A, superficial, B-F) fibres; lateral view slice and insets of stack, anterior to left, dorsal to top. (A) MyHC is absent from fast fibres (asterisk) and concentrated at fibre ends in slow fibres (arrowhead) in morphants, whereas actin is diffusely dispersed in cytoplasm of both fibre types (arrows). Blue dashes indicate somite borders. (B-D) z-line components α-actinin and titin and thin filament protein α-tropomyosin are concentrated in cytoplasmic puncta (arrowheads) and at fibre termini (arrows) after Mef2 knockdown. (E,F) M-line component MyBP-C is absent from residual myofibrils in morphants with regular sarcomere z-line spacing (arrowheads). Scale bars: 20 μm. PHENOTYPE:
|
|
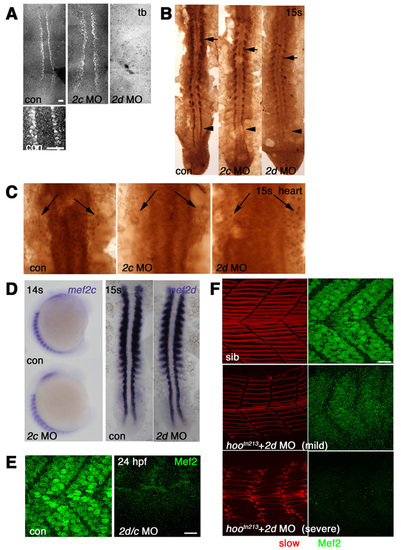
Morpholino knockdown of Mef2s. Immunodetection with anti-Mef2 antibody visualized with fluorescent (A,E,F) or DAB staining (B,C) or in situ mRNA hybridisation (D) viewed in dorsal flatmount (A-C,D right; anterior to top), lateral wholemount (D left; anterior to top, dorsal to left) or lateral flatmount (E,F, anterior to left, dorsal to top). (A) Adaxial cells in presomitic mesoderm show nuclear staining that is abolished by injection of mef2d MO, but not mef2c MO. (B) At 15 somites, mef2d MO ablates adaxial expression (arrowhead) and reduces expression in differentiated slow fibres (arrows), whereas mef2c MO has little effect. Note that mef2d-specific MO had no effect on anti-Mef2c signal (compare to Fig. S2B). (C) Heart anlagen in midline beneath neural tube shows nuclear reaction (arrows) in control that is abolished by mef2c MO, but not by mef2d MO. (D) At 14-15 somites, neither mef2d nor mef2c MOs affect levels of the cognate mRNA. (E) At 24 hpf, Mef2 immunoreactivity present in fibre nuclei is abolished by mef2d/c MO. (F) Confocal stack of Mef2 immunoreactivity (green) and slow MyHC (red) in embryos from a hoover heterozygote cross. mef2d MO-injected embryos show three phenotypes: severely affected with no detectable Mef2 (bottom), mildly affected with weak Mef2 (middle), or indistinguishable from uninjected control embryos, all of which contain strong Mef2 and well-organised slow fibres (top). Scale bars: 20 μm. EXPRESSION / LABELING:
|
|
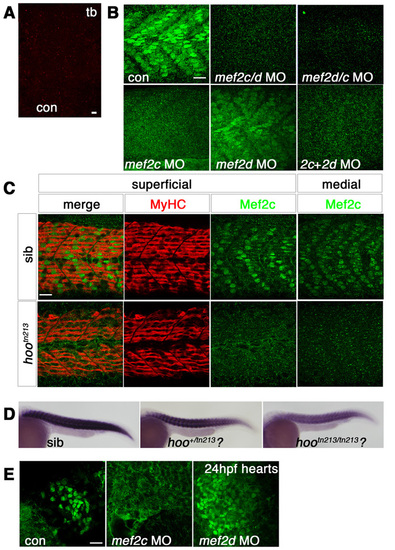
Morpholino knockdown of mef2c. Immunofluorescent detection of anti-Mef2c antibody (A, red; B-D, green) or slow MyHC (C, red) in skeletal muscle viewed in dorsal (A, presomitic mesoderm, anterior to top) or lateral (B,C, anterior to left, dorsal to top) flatmount. (A) Confocal stack showing unstained adaxial cells in presomitic mesoderm at tailbud stage. (B) At 24 hpf, confocal stacks reveal that Mef2c immunoreactivity is predominantly nuclear in both fast and slow muscle fibres. mef2c MO ablates nuclear signal, whereas mef2d MO has little effect. mef2c/d, mef2d/c and mef2c + mef2d MOs also ablate Mef2c immunoreactivity. (C) Single confocal slices of 24-hpf embryos from a cross of hoover heterozygotes. Mef2c immunoreactivity is ablated in superficial slow and medial fast fibres of 13/48 (27%) hootn213 mutant embryos, but slow muscle is unaffected. (D) mef2c mRNA is reduced in putative hootn213 mutants (21/67, 31%) and partially reduced in heterozygotes (27/67, 40%). (E) At 24 hpf, Mef2c immunoreactivity is predominantly nuclear in cardiomyocytes. mef2c MO ablates nuclear signal, whereas mef2d MO has no effect. Note that mef2c-specific MO greatly reduced anti-Mef2c stain in heart and muscle and anti-Mef2 in heart without diminishing muscle anti-Mef2 signal or cognate mRNA (compare to Fig. S1A-C). Scale bars: 20 μm. |
|
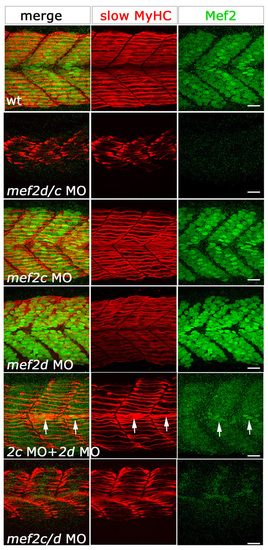
Several morpholino combinations produce the same skeletal muscle phenotype. Confocal stacks of fluorescent immunodetection of slow MyHC (F59, red) and anti-Mef2 (green) antibodies of 24-hpf somites of control and embryos injected with different MO combinations, viewed in lateral flatmount. Phenotype severity in slow muscle fibres correlates with levels of Mef2 in fibre nuclei. Single morpholinos show no detectable defects in slow fibres and wild-type levels of Mef2 in nuclei. Double knockdown with either mef2d/c MO, mef2c+mef2d MOs or mef2c/d MO, shows a similar fibre phenotype. Note the correlation of residual Mef2 (arrows) with better MyHC accumulation. Scale bars: 20 μm. EXPRESSION / LABELING:
|
|
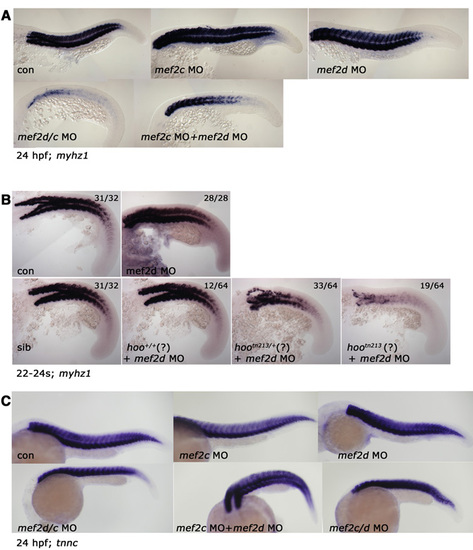
Mef2 knockdown reduces myhz1 but not tnnc mRNA in fast muscle. In situ mRNA hybridisation for myhz1 (A,B) and tnnc (C) (lateral view, anterior to left, dorsal to top). (A) Expression of myhz1 at 24 hpf in wild-type embryos injected with the indicated MOs. (B) myhz1 mRNA in wild type (top row) or embryos from a hoo heterozygote cross (bottom row) injected with mef2d MO. In wild type, mef2d MO had no effect, whereas <25% of the hoo lay appeared wild type, <50% mildly affected and <25% severely affected. (C) No change in level of tnnc in 24-hpf embryos injected with the indicated MO combinations. EXPRESSION / LABELING:
|

Unillustrated author statements EXPRESSION / LABELING:
|