- Title
-
The zebrafish candyfloss mutant implicates extracellular matrix adhesion failure in laminin {alpha}2-deficient congenital muscular dystrophy
- Authors
- Hall, T.E., Bryson-Richardson, R.J., Berger, S., Jacoby, A.S., Cole, N.J., Hollway, G.E., Berger, J., and Currie, P.D.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
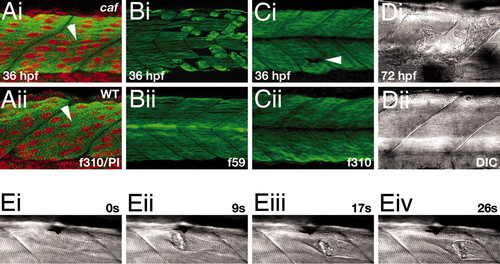
Initial muscle formation and degeneration in caf embryos. (A) Myoblasts fuse and elongate to form multinucleate fibers (arrowheads) that span the myotome between vertical myosepta. Single confocal scan through epaxial somite at 36 hpf. Green, f310 anti-fast MyHC; red, propidium iodide. (B) Degeneration in the slow layer, which is the first to elongate and fuse. Confocal projection of somite at 36 hpf. f59 anti-slow MyHC. (C) Degeneration in the deeper fast muscle layer. Arrowhead indicates a site of fiber loss. Confocal projection of somite at 36 hpf, f310 anti-fast MyHC. (D) Myotomal lesions at 72 hpf. DIC brightfield image of epaxial somite. (E) Stills from a time-lapse DIC movie showing fiber detachment and retraction in a homozygous caf embryo at 72 hpf, captured by using the anesthetic recovery technique. Time is indicated in seconds. The full movie sequence is available as SI Movie 1. In all images, anterior is to the left. PHENOTYPE:
|
|
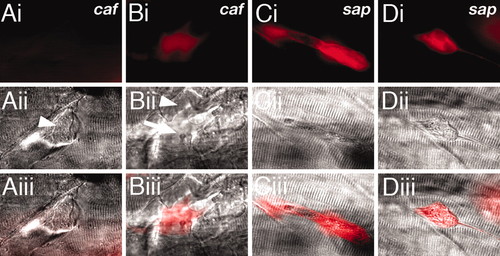
Unlike in dystrophin-deficient sap embryos, detached fibers in caf embryos do not show uptake of EBD, indicating that the sarcolemma is not compromised. (A) EBD within caf homozygotes is not taken up by a recently detached fiber in a homozygous caf embryo (arrowhead). (B) EBD can be seen to pool in a large myotomal lesion created by multiple retracting fibers (arrowhead). Retracted fibers themselves remain impermeable to EBD (arrow). (C) Damaged fibers in homozygous sap embryos are infiltrated by EBD before detachment. (D) EBD-positive retracted fiber in a sap embryo. (i) fluorescence, (ii) DIC, and (iii) merge. All images represent 72-hpf embryos, lateral views with anterior to the left. PHENOTYPE:
|
|
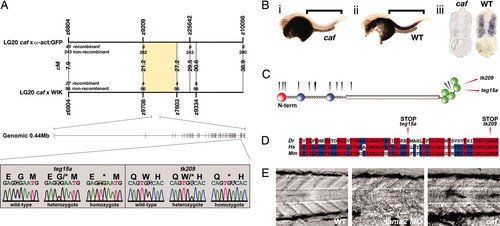
The caf phenotype is a result of mutations in the zebrafish homologue of the LAMA2 gene. (A) Initial bulked segregant analysis implicated markers z6804 and z10056 as being linked to the caf phenotype. Further fine mapping resolved flanking markers z9708 and z7603, delineating a candidate genomic region of H0.89 Mb, containing sequence with homology to human LAMA2. Single base changes in both cafteg15a and caftk209 were found in the homologue of human exon 60, resulting in the introduction of premature stop codons to the zebrafish lama2 ORF. (B) In situ hybridization to the lama2 mRNA at 48 hpf shows expression in the myotomal muscle. Strikingly, there is a severe reduction of message in caf homozygous embryos, suggestive of nonsense-mediated decay. Brackets indicate region of myotome. (i) Homozygous caf embryo, (ii) wild-type embryo, and (iii) transverse methacrylate sections through the trunk of caf (Left) and wild-type (Right) embryos. (C) Structure of the human LAMA2 protein. The positions of mutations that result in the MDC1A disease are indicated by black arrowheads. The homologous positions of the cafteg15a and caftk209 stop codons are indicated by red arrowheads. Both caf mutations occur in close proximity to known human MDC1A mutations, within the globular domain, which is essential for dystroglycan binding. Domains represented are laminin VI (red), laminin IV (blue), EGF-like (gray), and globular (green). (D) Amino acid alignment between zebrafish (Dr), human (Hs), and mouse (Mm) sequences for the mutant exon. Both cafteg15a and caftk209 mutations occur within completely conserved amino acids. (E) Injection of antisense morpholino oligonucleotides against the lama2 mRNA phenocopies the caf homozygous mutants. Seventy-two hpf, lateral view, anterior to the left. EXPRESSION / LABELING:
PHENOTYPE:
|
|
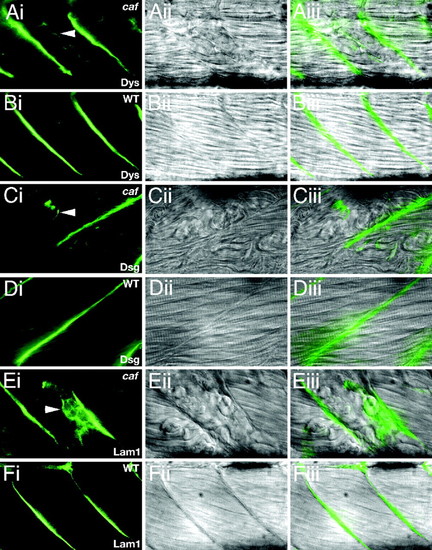
Immunohistochemistry at 72 hpf shows retraction of epitopes with detaching fibers, demonstrating that failure occurs within the extracellular matrix rather than at the sarcolemma. (A) caf, anti-dystrophin. (B) Wild-type anti-dystrophin. (C) caf, anti-β-dystroglycan. (D) Wild-type anti-β-dystroglycan. (E) caf, anti-laminin 1. (F) Wild-type anti-laminin 1. (i) Flourescence, (ii) DIC, and (iii) merge. Arrowheads indicate retraction of epitopes with individual fibers. Images show lateral views, anterior to the left. PHENOTYPE:
|
|
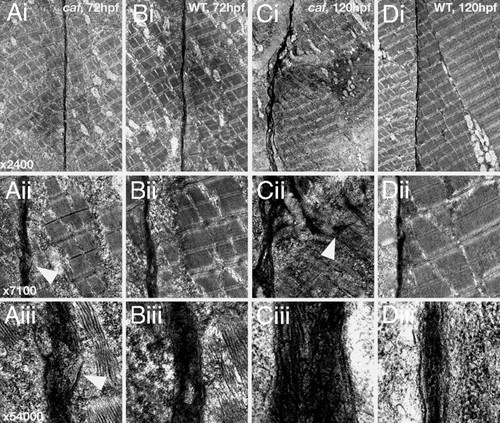
Transmission electron micrographs of the vertical myosepta in caf and wild-type embryos at 72 and 120 hpf. (A) caf, 72 hpf. The extracellular matrix phenotype is subtle and under low magnification (i; ×2,400) the myosepta appear normal. However, under higher magnification (ii; ×7,100 and iii; ×54,000), tearing of the myosepta can be seen that is not apparent in wild-type siblings (arrowheads). (B) Wild-type, 72 hpf. (C) caf, 120 hpf. Even under low magnification, the myosepta are grossly distorted and fibrotic. Under medium magnification, portions of extracellular matrix can be seen to infiltrate the myotome (arrowhead), pulled with the detaching fibers. Under high magnification, the myosepta are greatly increased in diameter and show condensed collagen fibers, indicative of a fibrotic response. (D) Wild-type, 120 hpf. PHENOTYPE:
|
|
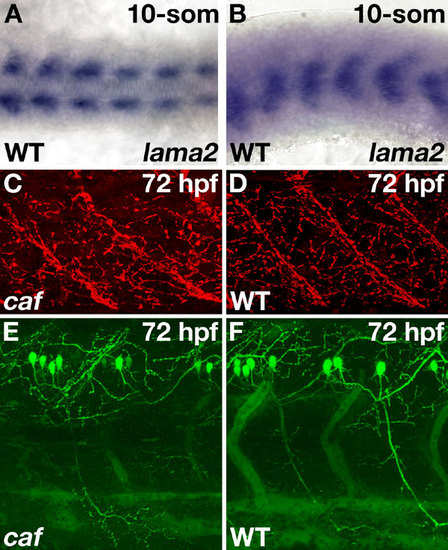
Expression of lama2 mRNA in the adaxial cells of a wild-type embryo and neuromuscular junction formation and primary motor neuron outgrowth. (A) In situ hybridization to Lama2 mRNA shows initial expression in the adaxial cells at the 10-somite stage. The adaxial cells are the first muscle cells of the embryo to differentiate. Wild-type, dorsal view, anterior to the left. (B) Wild type, lateral view, anterior to the left. (C) Staining with rhodamine-conjugated bungarotoxin allows visualization of the neuromuscular junctions (NMJs). No differences in the extent of NMJ formation were seen between caf embryos and wild-type siblings. Panels show confocal projections, lateral view, anterior to the left. Full projections are available as SI Movies 2 and 3. (D) Crossing caf zebrafish with the Tg(cmet:EGFP)ed6 line allowed visualization of the primary motor neurons. No defects in primary motor neuron formation or outgrowth were seen in caf embryos compared to wild-type siblings. Panels show confocal projections, lateral view, anterior to the left. Full projections are available as SI Movies 4 and 5. EXPRESSION / LABELING:
PHENOTYPE:
|