- Title
-
Autonomous and nonautonomous functions for Hox/Pbx in branchiomotor neuron development
- Authors
- Cooper, K.L., Leisenring, W.M., and Moens, C.B.
- Source
- Full text @ Dev. Biol.
|
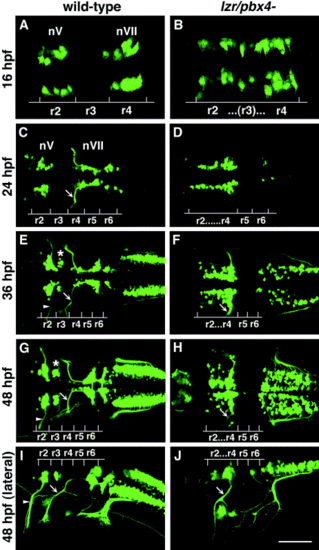
Confocal images of Isl1-GFP expression in live wild-type (left column) and lzr/pbx4-/- (right column) embryos. Anterior is to the left in all panels. All images are a dorsal view, except (I) and (J) which are lateral views. (A, B) The onset of GFP expression in trigeminal (nV) motor neurons in r2 and facial (nVII) motor neurons in r4 occurs at 16 hpf. In lzr/pbx4-/- embryos, motor neurons differentiate prematurely in r3. (C, D) By 24hpf in wild-type embryos, nVII cell bodies have migrated into r5 and r6, and axons leave r4 (arrow). In lzr/pbx4-/- embryos, presumptive nVII cells have not migrated posteriorly. (E, F) nV motor neurons in r3 appear by 36 hpf in wild-type embryos (asterisk). Arrows mark the nVII motor nerve exiting in r4; arrowhead in (E) marks the nV motor nerve exiting in r2. (G, H) By 48 hpf in wild-type embryos, nVII motor neurons have completed their migration into r6 and r7, while in lzr/pbx4-/- embryos, presumptive nVII motor neurons remain in r4. Labeling is as in (E) and (F). (I, J) Lateral views of 48-hpf embryos show a strong reduction of the nV nerve (arrowhead in I) in lzr/pbx4-/- embryos accompanied by a thickening of the nVII nerve (arrow in I, J). Scale bar, 50 μm in (A) and (B); 100 μm in (C–J). |
|
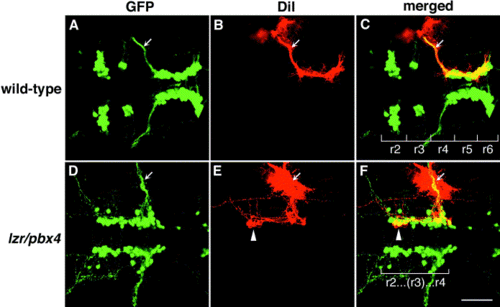
Motor axons from r2 and r3 of lzr/pbx4-/- embryos misroute through the r4 exit point with the facial (nVII) nerve. Anterior is to the left in all panels. DiI was applied to the nVII nerve root distal to its r4 exit point (arrow) in fixed, 36-hpf embryos to retrogradely label nVII cell bodies within the hindbrain. (A–C) DiI fills cells located primarily in r5 and r6 of wild-type embryos. (D–F) DiI applied to the nVII root of lzr/pbx4-/- embryos fills cells in r4 as well as cells located in r2 and r3 (arrowhead in E and F). Scale bar, 50 μm. |
|
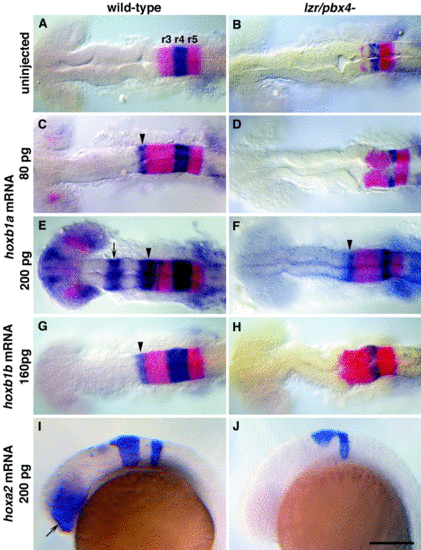
lazarus/pbx4 is required for the ectopic effects of hoxb1a, hoxb1b, and hoxa2. RNA in situ hybridization with krox20 (red in A–H, blue in I, J) and hoxb1a (blue in A–H) on wild-type (left column) and lzr/pbx4-/- (right column) embryos treated as shown. (A, B) In uninjected embryos, krox20 is expressed in r3 and r5, and hoxb1a is expressed in r4. In lzr/pbx4-/- embryos, krox20 expression in r3 and hoxb1a expression in r4 are reduced. (C–F) The effects of ectopically expressing hoxb1a at 80 (C, D) and 200 pg (E, F) are suppressed in lzr/pbx4-/- embryos. Arrowheads and arrows indicate ectopic endogenous hoxb1a expression in r2 and in the midbrain, respectively. (G, H) Similarly, the effects of over-expressing hoxb1b at about 160 pg are suppressed in lzr/pbx4-/- embryos. (I, J) Ectopic hoxa2 expression (200 pg) results in krox20 expression in the eyes of wild-type embryos (arrow in I), and this effect is also suppressed in lzr/pbx4-/- embryos. Scale bar, 100 μm in (A–H); 200 μm in (I) and (J). |
|
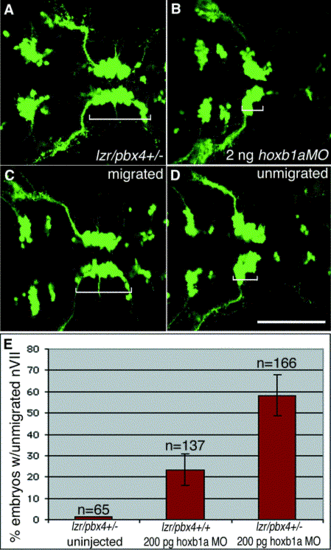
Loss of one copy of lazarus/pbx4 enhances effects of hoxb1a morpholinos on nVII motor neuron migration. (A–D) Confocal images of live embryos at 36 hpf. Brackets indicate the positions of facial (nVII) motor neurons in each panel. (A) lzr/pbx4+/- facial motor neurons migrate normally as in lzr/pbx4+/+ embryos (see Fig. 1E). (B) A total of 2 ng of hoxb1aMO blocks presumptive facial motor neuron migration in 95% of injected, lzr/pbx4+/+ embryos. (C, D) Classes of phenotypes observed after injecting 10-fold less (200 pg) hoxb1a morpholino: (C) “migrated” and (D) “unmigrated.” (E) A summary of the average percentage of lzr/pbx4+/+ and lzr/pbx4+/- embryos with unmigrated r4-derived motor neurons following injection of 200 pg hoxb1aMO. Error bars represent standard deviations across five independent experiments. The value “n” indicates the total number of embryos represented in these experiments. Chi-square tests on data from individual experiments demonstrate statistical significance to the differences observed between lzr/pbx4+/+ and lzr/pbx4+/- embryos (P < 0.01). Scale bar, 100 μm. |
|
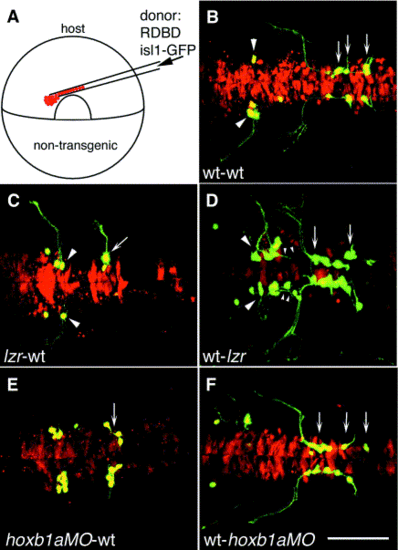
lazarus is required non-autonomously for nV axon pathfinding, and lazarus and hoxb1a are both required autonomously for nVII motor neuron migration. (A) Schematic of experimental design: cells from a lineage-labeled transgenic donor embryo were transplanted into the presumptive ventral hindbrain of an unlabeled, nontransgenic host embryo at 6 hpf. (B–F) Confocal images of 36-hpf host embryos. Anterior is to the left. (B) Control transplant of wild-type donor cells (red, yellow if GFP-expressing) into a wild-type host, showing normal development of donor-derived branchiomotor neurons. Arrowheads: trigeminal (nV) motor neurons in r2; arrows: facial (nVII) motor neurons in r5, r6, and r7. (C) lzr/pbx4-/- nVII motor neurons (arrow) fail to migrate out of r4 in a wild-type host, while lzr/pbx4-/- nV motor neurons (arrowheads) always extend axons correctly from lateral r2 when placed in a wild-type embryo. (D) In the reciprocal experiment, wild-type nVII motor neurons in a lzr/pbx4-/- host migrate out of r4 and into more posterior rhombomeres (arrows), while wild-type nV axons (small arrowheads) often misroute from r4 with the nVII nerve. (E) Presumptive nVII motor neurons from a hoxb1aMO-injected donor fail to migrate in a wild-type host. (F) Wild-type nVII motor neurons migrate posteriorly in a hoxb1aMO-injected host. Scale bar, 100 μm. |
Reprinted from Developmental Biology, 253(2), Cooper, K.L., Leisenring, W.M., and Moens, C.B., Autonomous and nonautonomous functions for Hox/Pbx in branchiomotor neuron development, 200-213, Copyright (2003) with permission from Elsevier. Full text @ Dev. Biol.