- Title
-
The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio)
- Authors
- Piotrowski, T. and Nüsslein-Volhard, C.
- Source
- Full text @ Dev. Biol.
|
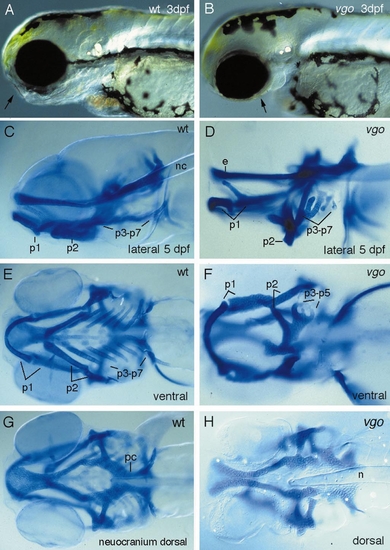
Photographs of live (A, B) and Alcian blue-stained wildtype (C, E, G) and vgo (D, F, H) larvae. A lateral view (B) of a live 3-dpf vgo larva shows that the pharyngeal arches are reduced and that the ear vesicle is much smaller. In vgo the pharyngeal cartilages fuse (p2–p7, D, F) and form one cartilaginous element ventrally (F), instead of being separate as in wild-type larvae (E). Also the most posterior cartilages are often reduced or absent (D, F). A dorsal view of the neurocranium (G, H) shows that the notochord (n) reaches farther anterior into the head in vgo (H). This defect seems to be caused by aberrant growth processes in the head, as at 1 dpf the notochord still has its wild-type position. Also, the mesodermally derived parachordalia (pc) are severely malformed. PHENOTYPE:
|
|
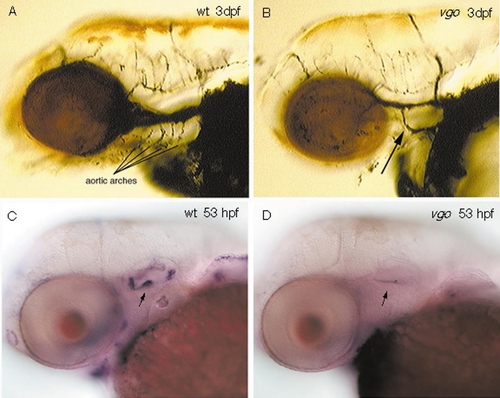
Distribution of aortic arches in 3-dpf wild-type (A) and vgo (B) larvae. Ink was injected into the blood stream to visualize the vascular system. In vgo (B) only one aortic arch is present (arrow), in contrast to at least four or five in the wild-type sibling (A). The bmp-4-positive sensory christae in the inner ear of 53-hpf wild-type embryos (C, arrow) are absent in vgo mutant embryos (D, arrow). EXPRESSION / LABELING:
PHENOTYPE:
|
|
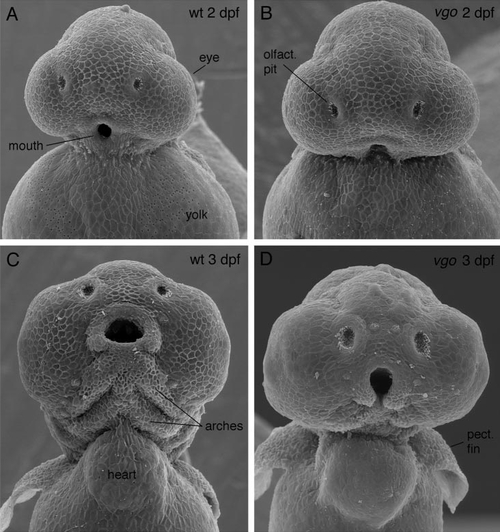
Scanning electron micrographs of 2- (A, B) and 3-dpf (C, D) vgo mutant larvae and their siblings. In 2-dpf wild-type larvae (A) the mouth has formed, whereas in vgo tissue ventral to the mouth is missing (B). In 3-dpf vgo larvae (D) no pharyngeal arches are visible and the mouth is not closed ventrally as in the wild-type siblings (C). PHENOTYPE:
|
|
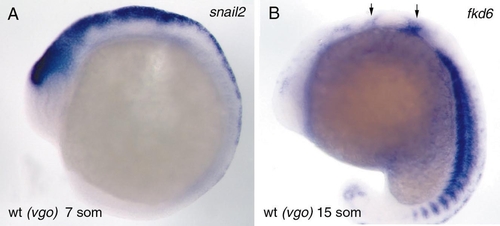
The expression pattern of snail 2 at the 7-somite stage in premigratory neural crest is indistinguishable between wild-type and vgo embryos (A). During early stages of neural crest migration (15 somites) the expression of fkd-6 is also normal in vgo, indicating that the number of neural crest cells and their migration anterior and posterior to the ear (arrows) is unaltered in vgo embryos at this level of detection (B). EXPRESSION / LABELING:
PHENOTYPE:
|
|
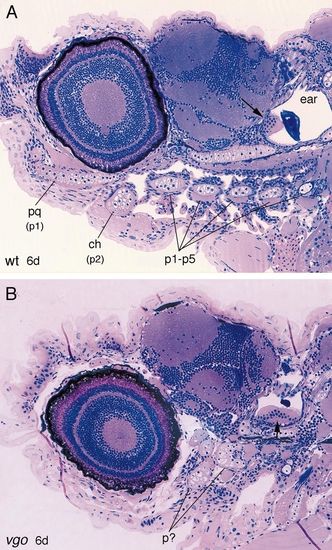
2-μm-thick sagittal sections through 6-dpf wild-type (A) and vgo mutant larvae (B). In wild-type larvae the five posterior pharyngeal arches (p1–p5) are separated from one another by the gill clefts, whereas in vgo no organization of the arches is recognizable. Also the ear in vgo is much smaller, although the sensory patches (maculae, arrows) underlying the otoliths are present. Abbreviations: pq, palatoquadrate (dorsal element of first pharyngeal arch); p1, p2, pharyngeal arches 1 and 2; ch, ceratohyal (element of second pharyngeal arch). PHENOTYPE:
|
|
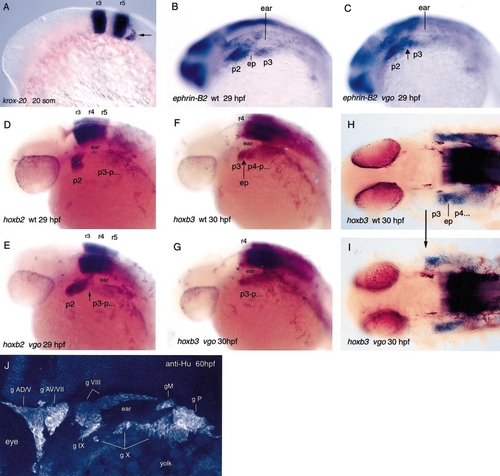
In situ hybridization with krox-20 (A), ephrin-B2 (B, C), hoxb2 (D, E), and hoxb3 (F–I), which are segmentally expressed in the hindbrain. In 20-somite-old vgo mutant embryos krox-20 is normally expressed in rhombomeres 3 and 5 (A), as well as in the neural crest cells that migrate from rhombomere 5 into pharyngeal arches 3–7 posterior to the ear (arrow). These cells contribute to the third neural crest stream. At this stage the posterior endodermal pouches have not formed yet and therefore the pharyngeal arches 3–7 cannot be distinguished. The expression patterns of hoxb2 in r3, r4, and r5 in 29-hpf wild-type (D) and vgo embryos (E) are indistinguishable from each other, indicating that hindbrain segmentation is not affected. However, in vgo (E) the neural crest cells that have migrated from r4 and r7 into the second (p2) and more posterior arches spread anteriorly along the A-P axis. In the pharyngeal arch region, r7-derived neural crest cells contact r4-derived neural crest cells (arrow), indicating that the arches are not separated from each other. hoxb3 is expressed in the third neural crest stream that migrates into p3 and the more posterior arches (F, H). No endodermal pouches form within the hoxb3 expression domain in vgo (F, G, arrow; ep, endodermal pouch). The expression is anteriorly expanded in vgo and is adjacent to the posterior expression boundary of hoxb2 in p2 (H, I, arrow). The sharp anterior expression boundary of hoxb3 suggests that neural crest cells from p2 and p3 do not intermingle (I). The sensory cranial ganglia develop normally in 60-hpf vgo as visualized with an antibody against Hu proteins that are expressed in neurofilaments (J). Sensory cranial ganglia possess a neural crest contribution, suggesting that in vgo the neural crest cells are correctly specified and migrate to their proper targets. The nomenclature of Raible and Kruse (2000) and Piotrowski and Northcutt (1996) was adopted. Abbreviations: g AD, anterodorsal lateral line ganglion; g AV, anteroventral lateral line ganglion; g M, middle lateral line ganglion; g P, posterior lateral line ganglion; g V, trigeminal ganglion; g VII, facial sensory ganglion; g VIII, octaval ganglion; g X, vagus ganglion. EXPRESSION / LABELING:
|
|
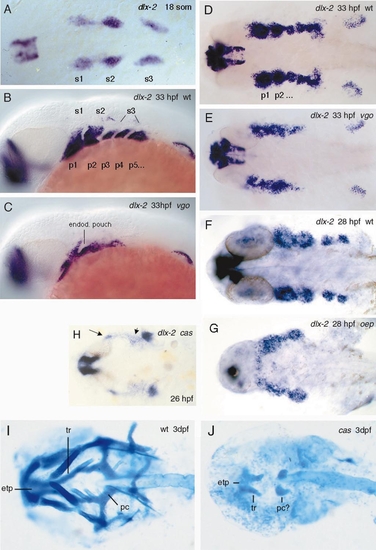
(A–G) In situ hybridization with dlx-2, a gene which is expressed in neural crest cells, nuclei in the forebrain, and pectoral fins. At 18 somites dlx-2 is expressed in three neural crest streams (A, s1, s2, s3, dorsal view). In 33-hpf wild-type larvae (B, lateral view; D, dorsal view) neural crest cells have populated the arches, which are separated from each other by the endodermal pouches (nonstaining tissue between the arches). Arches p5–p7 have not been divided by the endoderm yet. At this stage the endodermal pouches consist of two rows of cells which have not formed a cavity yet. In vgo (C) only the first endodermal pouch is present. In the absence of posterior endodermal pouches the neural crest cells of the posterior pharyngeal arches fuse (C, E). (F and G) Dorsal views of dlx-2 expression in 28-hpf wild-type and oep mutant embryos, respectively. (H) dlx-2 expression pattern in endodermal-deficient cas mutants. No distinct pharyngeal arches form and the neural crest cells migrate to ectopic locations (arrows). In oep (G) and cas (H), neural crest cells of the anterior arches fuse with each other and expression is reduced in the posterior arches. (I, J) Alcian blue-stained cartilage preparations of 3-dpf wt (I) and cas (J) larvae. In cas larvae the cranial cartilages are severely reduced. The only elements present are the anterior parts of the neurocranium (reduced trabeculae (tr) and ethmoid plate (etp)) and remnants of the parachordals (pc) or possibly two pharyngeal cartilages of unknown identity. Abbreviations: s1–s3, neural crest streams 1–3; p1–p7, pharyngeal arches 1–7. EXPRESSION / LABELING:
PHENOTYPE:
|
|
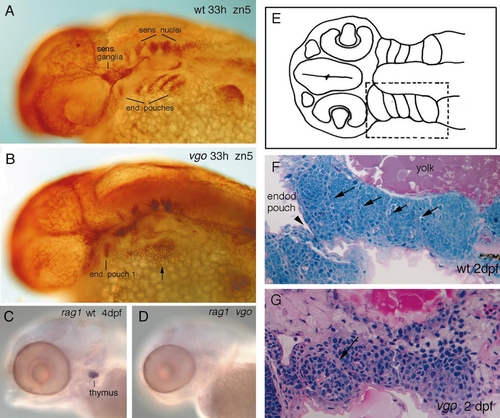
33-hpf wild-type sibling (A) and vgo mutant (B) stained with the antibody Zn-5. Zn-5 stains the endoderm of the pharyngeal pouches (prospective gill slits), as well as sensory neurons and ganglia of the nervous system. In the wild-type embryo five pouches are visible; the most posterior pouches have not formed yet (A). In vgo mutant embryos only the first endodermal pouch is visible and dispersed staining can also be observed ventral to the ear (B, arrow). However, these Zn-5-positive cells do not show any sign of segmental organization. Other endodermal pouch derivatives, such as the thymus, are absent in vgo mutants, as revealed by in situ hybridization with rag-1 (C, D) of 4-dpf embryos. The lack of organization of the endodermal pouches can also be observed in 2-μm-thick horizontal plastic sections of 2-dpf wild-type (F) and vgo (G) embryos. (E) A schematic drawing of a section to show the location of the developing pharyngeal arches that are magnified in (F) and (G). In the wild-type sibling (F) the pharyngeal arches p3–p7 are separated from one another by two rows of endodermal cells (arrows). At this stage, only the endodermal pouch between the second and the third arches has pierced through the ectoderm and thus has formed a gill slit (arrowhead). In the vgo larvae (G) the second arch has formed properly but no organization of the posterior arches can be observed. |
|
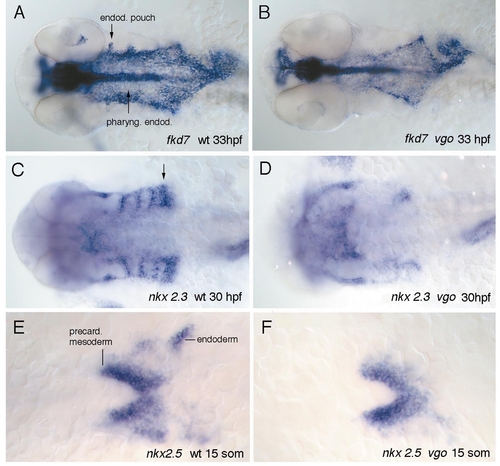
Whole-mount in situ hybridization of wild-type (A) and vgo (B) embryos with fkd-7. At 33 hpf fkd-7 is expressed not only in the medial pharyngeal endoderm but also in the developing pouches (A, arrows). The expression pattern of fkd-7 in vgo within the medial endoderm (B) indicates that the reduction of the pouches is not caused by a general reduction of the endoderm, even though the expression domain is shorter than in wild-type siblings. (C–F) Ventral views of expression patterns of nkx2.3 and nkx2.5 in 30-hpf and 15-somite wild-type (C, E) and vgo mutant larvae (D, F) (anterior to the left). nkx2.3 is expressed in the endodermal pouches in a pattern complementary to that of dlx-2 (Fig. 8C). At this stage the posterior pouches are not separated from each other and appear as a thicker patch of staining (C, arrow). In vgo expression in the endodermal pouches is very much reduced, although it is present in the most anterior pouches (D). At 15 somites nkx2.5 is expressed in precardiac mesoderm as well as in prospective anterior endoderm (E). In vgo the expression in the precardiac mesoderm is normal, whereas the endodermal expression is absent (F). The reduction of nkx2.5 in the endoderm is the earliest defect observed in vgo. EXPRESSION / LABELING:
|
|
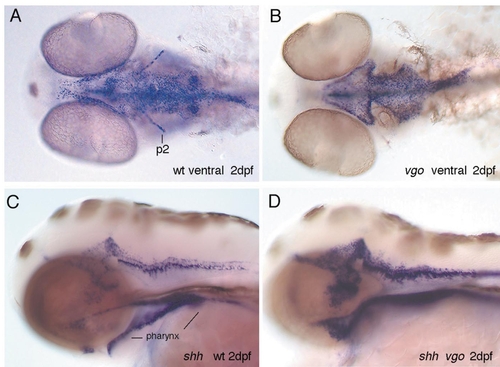
In 2-dpf wild-type larvae shh is expressed in the endoderm, floor plate, and brain, as well as in the second arch (A, C). Ventral views of wild-type (A) and vgo (B) embryos show that the endodermal expression domain of shh is shortened along the A-P axis in vgo and that in some vgo embryos expression in the second pharyngeal arch (p2) is absent. Lateral views of wild-type (C) and vgo (D) embryos show that in vgo the pharynx does not form a tube but remains a thin layered sheet of cells. |
|
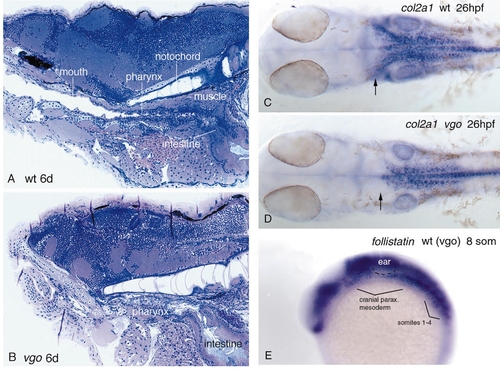
Mesodermal defects in vgo. Sagittal sections through a 6-dpf wild-type sibling (A) and a vgo mutant larvae (B). In the wild-type larva the mouth forms a connection with the pharynx and the intestine (A). In vgo (B), the intestine develops normally but the pharyngeal endoderm does not form a tube and no connection between the mouth and the digestive tract exists. Muscles ventral to the notochord in the dorsal roof of the pharynx are also very much reduced compared to wild type. In vgo the notochord reaches farther anterior than in wild-type siblings, which is caused by a reduction in the growth rate of the pharyngeal region. (C and D) 26-hpf embryos stained with col2a1. In vgo (D) the mesenchyme that gives rise to the parachordalia of the neurocranium is reduced (arrows), whereas the anterior tip of the notochord is still at the same position as in the wild-type siblings. (E) The cranial paraxial mesoderm is not reduced in 8-somite vgo embryos as revealed by identical follistatin expression in all embryos of a clutch coming from vgo heterozygote parents. EXPRESSION / LABELING:
PHENOTYPE:
|
|
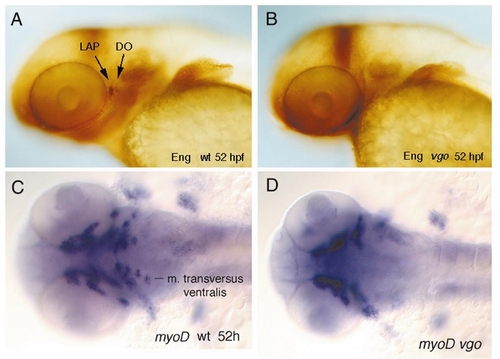
Whole-mount in situ hybridization experiments with genes that are expressed in the mesoderm. (A and B) Lateral views of two jaw muscles, the levator arcus palatini (LAP) and the dilator operculi (DO), that express Engrailed during development. (B) In 52-hpf vgo embryos these two muscles are absent, as revealed by antibody labeling with 4D9, which recognizes Eng. All other voluntary pharyngeal muscles express myoD. (C and D) Ventral views of myoD expression in 52-hpf wild-type (C) and vgo (D) embryos. In vgo embryos the muscles which are associated with the pharyngeal arches 4–7 do not express myoD or are absent. |

Unillustrated author statements |
Reprinted from Developmental Biology, 225(2), Piotrowski, T. and Nüsslein-Volhard, C., The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio), 339-356, Copyright (2000) with permission from Elsevier. Full text @ Dev. Biol.