- Title
-
Roles for Fgf signaling during zebrafish fin regeneration
- Authors
- Poss, K.D., Shen, J., Nechiporuk, A., McMahon, G., Thisse, B., Thisse, C., and Keating, M.T.
- Source
- Full text @ Dev. Biol.
|
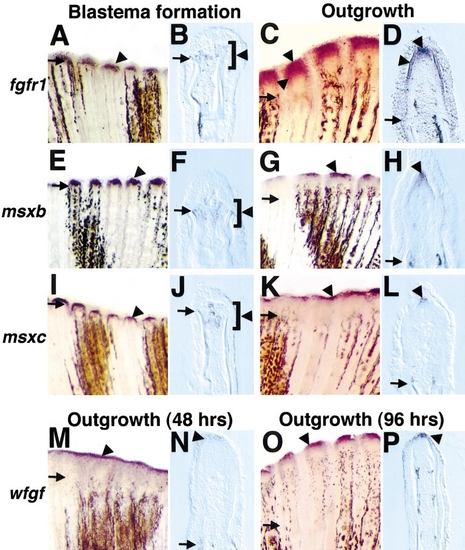
Fgfr1, msxb, msxc, and wfgf are expressed in the fin regenerate during blastema formation (18–24 h postamputation) and/or regenerative outgrowth (48 h postamputation unless otherwise indicated). (A) Fgfr1 expression at 24 h postamputation (violet stains indicate positive signal, arrowhead). Arrows demarcate amputation plane in each photograph. (B) Longitudinal section (bottom, anterior; top, posterior) through an 18-h fin stained for fgfr1. Mesenchymal cells located between and just distal to hemirays faintly expressed fgfr1 (brackets outline region of expression). (C) Fgfr1 expression at 48 h postamputation. (D) Section through a 48-h fin regenerate stained for fgfr1 mRNA expression. Two different domains are evident: one in mesenchymal cells representing the distal blastema and one expressing bilaterally in the basal layer of the regeneration epidermis. (E) Msxb expression at 24 h postamputation. (F) Longitudinal section of 18-h fin regenerate assessed for msxb expression, indicating msxb-positive cells between and extending beyond hemirays. (G) msxb expression at 48 h postamputation. (H) Section of 48-h fin regenerate labeled for msxb demonstrates expression in the distal blastema. (I) msxc expression at 24 h postamputation. (J) Longitudinal section of 18-h fin indicates msxc-positive cells between hemirays. (K, L) Expression of msxc at 48 h was virtually identical to that of msxb in whole mounts (K) and sections (L). (M) wfgf expression at 48 h postamputation. (N) Longitudinal section of fin stained for wfgf expression at 48 h. Only the distalmost cells of the regeneration epidermis expressed wfgf. (O) wfgf expression at 96 h postamputation. (P) Section of 96-h regenerate assessed for wfgf expression, indicating an epidermal hybridization signal. Original magnification in whole mounts (A, C, E, G, I, K, M, O) was 50x and was 250x in sections (B, D, F, H, J, L, N, P). |
|
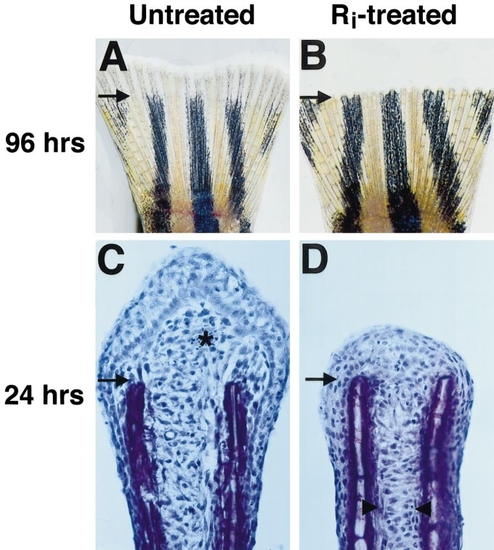
Fgfr1 inhibition blocks fin regeneration. (A) Fin from untreated fish at 96 h after amputation, showing normal regrowth and new segmentation. Arrows demarcate amputation plane in each photograph. (B) Fin from fish treated with Ri for 96 h immediately following amputation. These fins showed no new growth. Here, the amputated edge appears saw-toothed due to the retraction of tissue between rays. (C) Hematoxylin stain of 24-h fin regenerate section from untreated fish (asterisk denotes new blastema). (D) Fin regenerate section from fish treated with Ri for 24 h. Note the lack of blastema. However, Ri-treated fin regenerates showed mesenchymal disorganization (arrowheads mark boundary between organized and disorganized tissue), as well as longitudinal arrangement suggestive of migration. Original magnification in (A, B) was 20x and in (C, D) 400x. |
|
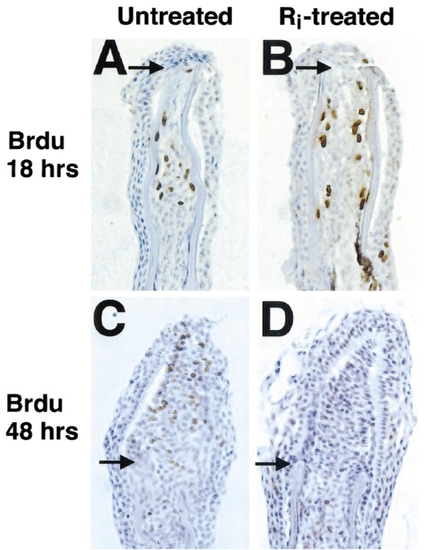
Fgfr1 inhibition blocks proliferation of established blastemal cells. (A, B) Section of 18-h fin regenerates indicating BrdU incorporation (that occurred during 12–18 h postamputation) in untreated (A) and Ri-treated (B) fins. Ri had no effects on BrdU incorporation in proximal mesenchymal tissue at this stage. Arrows demarcate amputation plane in each photograph. (C, D) Section of fins allowed to regenerate for 40 h prior to (C) a 2-h period without treatment before 6 h incubation with BrdU or (D) a 2-h Ri preincubation period before 6 h treatment with both Ri and BrdU. While proximal mesenchymal cell BrdU incorporation was normal during the brief Ri incubation, BrdU incorporation in distal blastemal cells was never observed, and incorporation in proximal blastemal cells was dramatically reduced. Original magnification was 400x. |
|
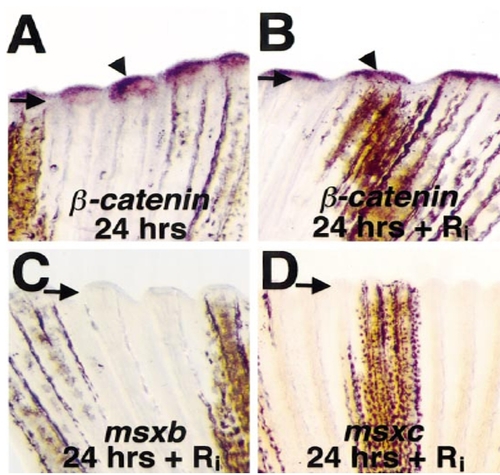
Fgfr1 inhibition blocks msx gene expression in the fin regenerate. (A, B) Fins from untreated fish (A) and Ri-treated fish (B) stained for β-catenin mRNA expression at 24 h postamputation (arrowheads mark signals). Arrows demarcate amputation plane in each photograph. (C, D) Fins from Ri-treated fish assessed for msxb (C) or msxc (D) expression at 24 h postamputation. Wound epidermal β-catenin expression was normal in Ri-treated fish, but expression of mesenchymal msxb and msxc was very low or absent (compare with Figs. 2E and 2I). Original magnification was 50x. |
|
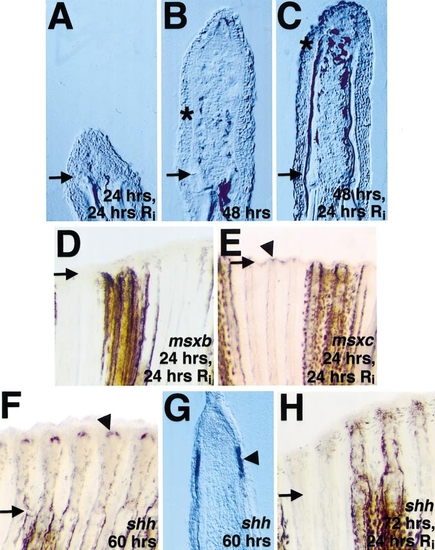
Fgfr1 inhibition blocks outgrowth and reduces msxb/c and shh expression in ongoing fin regenerates. (A) Section of amputated fin from fish allowed to regenerate for 24 h before a 24-h treatment with Ri. (B) Section of untreated fin at 48 h postamputation. (C) Section of fin from fish allowed to regenerate for 48 h before treatment with Ri. Note that little outgrowth occurred during Fgfr1 inhibition. By comparing (B) and (C), it is apparent that, during Ri treatment, melanocyte migration and bone matrix deposition (still inconspicuous in (B)) continued despite the lack of outgrowth. Asterisks denote distalmost points of bone deposition (C) and pigment cell localization (B, C), and arrows demarcate the amputation plane in each photograph. (D, E) Whole-mount views of fin allowed to regenerate for 24 h, treated with Ri, and assessed for msxb (D) or msxc (E) expression. Note that Ri treatment removed (D) or diminished (E; arrowhead marks signal) established expression (compare with Figs. 2E and 2I). (F) shh was expressed at 60 h postamputation in dual fin ray domains. (G) Section through fin shown in (F) indicates bilateral shh expression domains in the basal layer of the epidermis. (H) Fin allowed to regenerate for 72 h, treated with Ri, and stained for shh mRNA expression. shh transcripts were undetectable following Ri application. Original magnification in (A–C, G) was 250x and in (D, E, H) 50x. |
Reprinted from Developmental Biology, 222(2), Poss, K.D., Shen, J., Nechiporuk, A., McMahon, G., Thisse, B., Thisse, C., and Keating, M.T., Roles for Fgf signaling during zebrafish fin regeneration, 347-358, Copyright (2000) with permission from Elsevier. Full text @ Dev. Biol.