Fig. 6
- ID
- ZDB-FIG-220723-22
- Publication
- Hotz et al., 2021 - Loss of glutamate transporter eaat2a leads to aberrant neuronal excitability, recurrent epileptic seizures, and basal hypoactivity
- Other Figures
- All Figure Page
- Back to All Figure Page
|
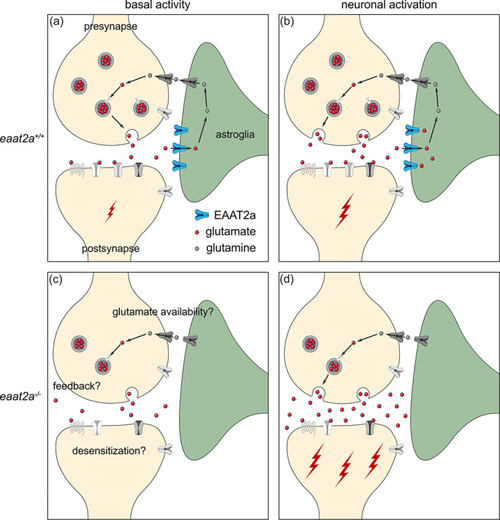
Working model of coexisting hyper‐ and hypoactivity in eaat2a −/− mutants. (a, b) Glutamate transporter EAAT2a is important for both clearance of glutamate at the synaptic cleft and recycling of glutamate to the presynapse during basal activity (a) and neuronal activation (b). (c) Loss of EAAT2a function leads to neuronal hypoactivity during basal periods, potentially reflecting reduced levels of available glutamate/glutamine. (d) Once glutamate is released in higher quantities from the presynapse (e.g., following light stimuli or spontaneous glutamate release), astroglia cannot sufficiently take up glutamate. Accumulated glutamate in the synaptic cleft hyperexcites postsynaptic neurons, which can lead to a cascade of glutamate release across the nervous system and results in epileptic seizures. Such recurrent epileptic seizures could also potentially lead to desensitization or a negative feedback to the presynapse (c), which can further explain reduced basal neuronal activity in eaat2a −/− mutants |