|
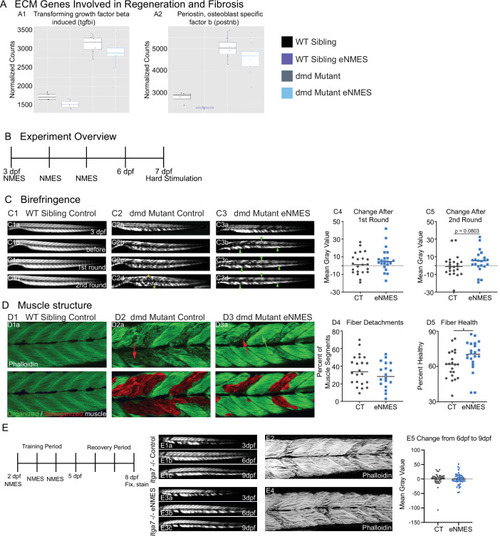
Muscle resilience to hard stimulation is increased with endurance neuromuscular electrical stimulation (eNMES), and Itga7 is required for eNMES-mediated improvement.We identified three extracellular matrix (ECM) genes from RNAseq analysis, tgfbi (A1), postnb (A2), itgb1b.2 (not shown) that are significantly upregulated in dmd mutants compared to WT siblings and trend towards being downregulated with eNMES in dmd mutants. (B) Experimental overview. At 3 days post-fertilization (dpf) (disease onset), birefringence images were taken followed by the first session of eNMES. At 4 and 5 dpf, zebrafish undergo the second and third NMES sessions, respectively. At 7 dpf, muscle resilience was tested using a hard electrical stimulation paradigm intended to cause muscle damage. (C) Birefringence images were taken at 3 dpf (C1a). (C1b–d) Birefringence images were taken at 7 dpf before the first hard stimulation (C1b), after the first hard stimulation (C1c), and after the second hard stimulation (C1d). No visible changes in birefringence are observed in WT siblings after the two stimulation sessions. (C2) For dmd mutant controls, the first round of stimulation did not result in visible changes to birefringence (C2c), but, after the second round, areas of muscle degeneration are visible (C2d, yellow asterisks). Conversely, in dmd mutants that completed three sessions of eNMES, the first (C3c) and second (C3d) rounds of stimulation did not result in visible changes to birefringence (green arrowheads denote intact areas of birefringence that remain intact). (C4, C5) Change in birefringence from before to after the first round (C4) and second (C5) of stimulation suggests that eNMES training may improve muscle resilience. (D) Phalloidin was used to visualize individual muscle fibers. (D1a) Representative image of a WT sibling control demonstrates healthy, organized muscle fibers, and myotomes. (D2a) Representative image of a dmd mutant control highlights disorganized and wavy muscle fibers and fiber detachments. (D3a) Representative image of a dmd mutant that completed eNMES demonstrates some wavy muscle fibers and detached fibers intermixed with relatively healthy myotomes. (D4) The percent of muscle segments with detached fibers following the hard stimulation is reduced in dmd mutants that complete eNMES training compared to dmd mutant controls. For this analysis, a muscle segment was defined as half of a myotome. (D1b, D2b, D3b) Machine learning was used to quantify muscle health pixel-by-pixel. Green indicates healthy pixels while red indicates unhealthy pixels. (D5) The percent of healthy muscle following the hard stimulation is significantly higher in dmd mutants that completed eNMES compared to dmd mutant controls. (E) itga7 mutants were subjected to the same eNMES protocol that results in improvements in dmd mutants. Note that eNMES does not improve birefringence (panels E1, E3, quantified in E5) or muscle structure in itga7 mutants (E2, E4). All data were analyzed using two-sided t-tests. *p<0.05.
|