Figure 7.
- ID
- ZDB-FIG-211120-30
- Publication
- Gui et al., 2021 - De novo identification of mammalian ciliary motility proteins using cryo-EM
- Other Figures
- All Figure Page
- Back to All Figure Page
|
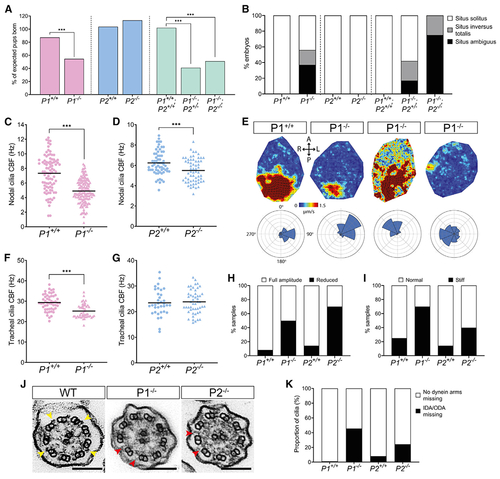
Pierce1-deficient mice have aberrant nodal and tracheal cilia motility
(A) Pierce1 (P1)−/− and P1−/−;P2−/− double knockout mice have high embryonic lethality, whereas P2−/− mice do not. All other genotypes were born at the expected frequencies. Chi-square analysis was used to evaluate significance. (B) P1−/− and P1−/−;P2−/− embryos have visceral organ situs defects. A bar chart shows the proportion of embryos displaying situs solitus (normal organ positioning), situs inversus totalis (total inversion to normal), and situs ambiguus (abnormal organ positioning) for each genotype at E13.5. P1+/+ (n = 16), P1−/− (n = 16), P2+/+ (n = 9), P2−/− (n = 10), P1+/+;P2+/+ (n = 7), P1−/−;P2+/− (n = 12) and P1−/−;P2−/− (n = 8) are shown. All other genotypes displayed only situs solitus. (C and D) Nodal cilia beat frequency (CBF) is reduced significantly in P1−/− and P2−/− embryos at E8.0. Average CBF is 7.3 Hz versus 4.9 Hz (P1+/+ versus P1−/−) and 5.9 Hz versus 5.1 Hz (P2+/+ versus P2−/−). Numbers of embryos analyzed are 19, 36, 9, and 8 for P1+/+, P1−/−, P2+/+, and P2−/−, respectively. CBFs of 5–10 cilia were quantified per node. ***p < 0.001 Student’s t test. Heterozygous genotypes did not show significant differences in their mean CBF. (E) Mean fluid velocity is reduced and/or directionality is abnormal in P1−/− embryonic nodes at E8.0. Dark red refers to high velocity (1.5 μm/s), and dark blue refers to low velocity (0 μm/s). Localized directionality of flow is shown by black arrows. Anterior (A), posterior (P), left (L), and right (R) axes are annotated. Overall directionality of flow is depicted in rose plots in the bottom panel; vector direction is indicated in 8 directional segments, with the number of vectors indicated by the size of the segment. P1+/+ (n = 8) embryos display an organized, leftward nodal fluid flow, whereas P1−/− (n = 8) embryos show a range of unusual phenotypes, including leftward flow (n = 2), disordered flow (n = 4), and weak flow with no overall directionality (n = 2) (panels from left to right). P1+/− did not differ compared with WT embryos. (F and G) Tracheal CBF is reduced in P1−/− but not P2−/− mice. Average CBF is 29.4 Hz versus 25.2 Hz (P1+/+ versus P1−/−) and 23.4 Hz versus 23.8 Hz (P2+/+ versus P2−/−). 7–11 trachea were harvested for each genotype, with 5 ring sections assessed per trachea. ***p < 0.001 Student’s t test. P2+/− mean CBF did not differ compared with WT embryos. (H and I) Tracheal cilia beat pattern is disrupted in P1−/− and P2−/− adult mice. Bar charts show the proportion of P1+/+ (n = 12), P1−/− (n = 10), P2+/+ (n = 7), and P2−/− (n = 10) trachea displaying cilia with a reduced beat amplitude (H) and a stiff waveform (I). P1+/− and P2+/− did not differ compared with the WT. (J) TEM of tracheal cilia cross-sections from WT, P1−/−, and P2−/− mice. Yellow arrows on the WT image indicate ODAs, whereas red arrows in the P1−/− and P2−/− images indicate missing dynein arm(s). Scale bars, 100 nm. (K) Quantification of dynein arm defects observed in adult mouse tracheal cilia from P1+/+ (n = 3,387 cilia), P1−/− (n = 4,597 cilia), P2+/+ (n = 3,604 cilia), and P2−/− (n = 3, 540 cilia) genotypes. ~46% of P1−/− and ~24% of P2−/− tracheal cilia axonemes have missing dynein arms. Micrographs were assessed by three independent evaluators blind to genotype. See also Figure S6, Table S3, and Videos S4 and S5. |
Reprinted from Cell, 184(23), Gui, M., Farley, H., Anujan, P., Anderson, J.R., Maxwell, D.W., Whitchurch, J.B., Botsch, J.J., Qiu, T., Meleppattu, S., Singh, S.K., Zhang, Q., Thompson, J., Lucas, J.S., Bingle, C.D., Norris, D.P., Roy, S., Brown, A., De novo identification of mammalian ciliary motility proteins using cryo-EM, 5791-5806.e19, Copyright (2021) with permission from Elsevier. Full text @ Cell