Fig. 1
- ID
- ZDB-FIG-180404-27
- Publication
- Sakamaki et al., 2015 - Conservation of structure and function in vertebrate c-FLIP proteins despite rapid evolutionary change
- Other Figures
- All Figure Page
- Back to All Figure Page
|
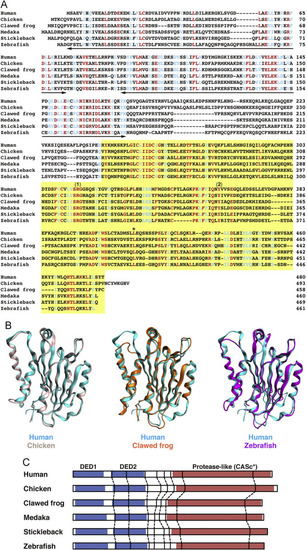
Analysis of the protein structures of c-FLIP proteins. (A) Multiple alignment of amino acid sequences of human, chicken, African clawed frog, medaka, stickleback, and zebrafish c-FLIP proteins. Identical and similar amino acids in all family members are indicated by red and blue, respectively. The two bold lines and a yellow box indicate the DED motif and the protease-like CASc* domain, respectively. The numbers (1) and (2) shown above the sequences indicate the crucial arginine and tyrosine amino acid residues, which are the cause of the deactivation of the protease activity [8], and an asterisk indicates the conserved leucine residue. (B) Structural superposition of human c-FLIP and non-mammalian c-FLIP proteins. Structural models of the CASc* domain of chicken (light pink), clawed frog (orange), and zebrafish (magenta) c-FLIP proteins were computationally generated and superimposed with the CASc* domain of human c-FLIP (cyan, PDB ID: 3H13). (C) Comparison of the exon-intron organization of the CFLAR genes. The splice junction sites of the CFLAR/c-FLIP genes in vertebrates are indicated by vertical lines. Their positions were defined by the comparison of the respective genomic and cDNA sequences from the species listed in Table A2. The regions corresponding to two DED motifs and a CASc* domain are indicated by blue and red boxes, respectively. |