- Title
-
Epicoccin A Ameliorates PD-like Symptoms in Zebrafish: Enhancement of PINK1/Parkin-Dependent Mitophagy and Inhibition of Excessive Oxidative Stress
- Authors
- Ye, H., Li, D., Zhang, L., Wang, Y., Wang, C., Jin, M., Lin, H., Li, P., Sun, C., Li, N.
- Source
- Full text @ Mar. Drugs
|
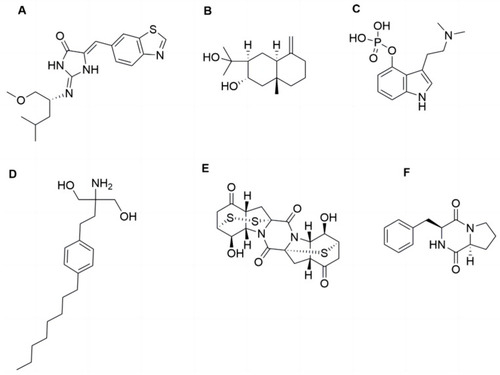
The structures of epicoccin A and the compounds mentioned. ( |
|
Remissive effect of epicoccin A on the loss of DA neurons in PD. ( |
|
The inhibitory effect of epicoccin A on MPTP-induced nervous system injury in zebrafish brains. ( |
|
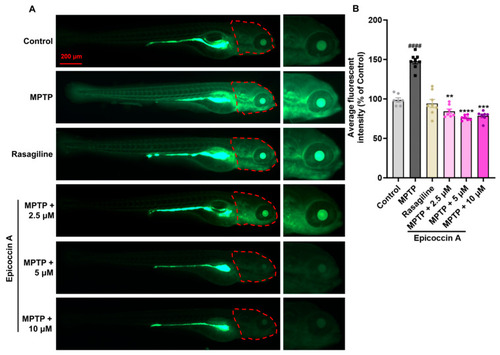
Ameliorative effect of epicoccin A on MPTP-induced loss and disorganization of neural vasculature. Representative fluorescent microscopy images of |
|
The inhibitory effect of epicoccin A on ROS overproduction in the brains of zebrafish with PD. ( |
|
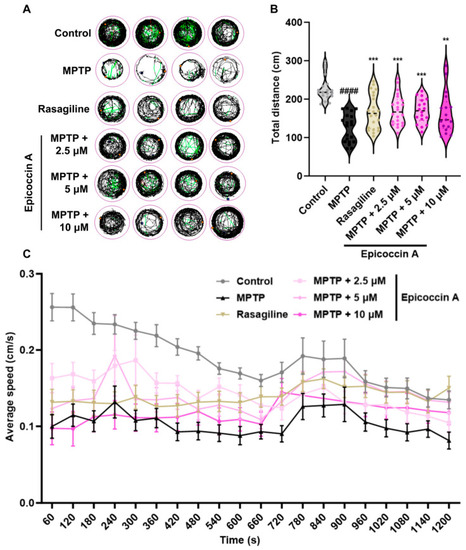
Improved effect of epicoccin A on MPTP-induced locomotor impairment in zebrafish. ( |
|
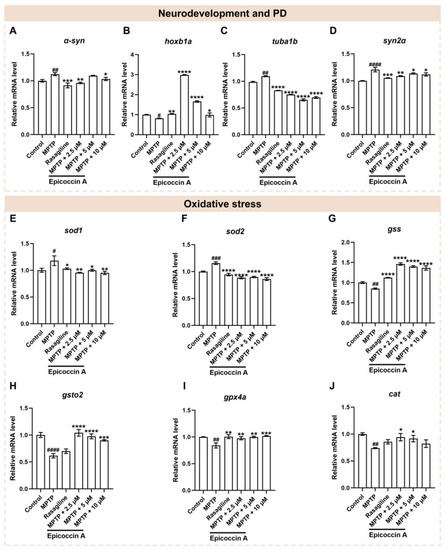
The mRNA expression levels of genes associated with PD, neurodevelopment, and oxidative stress. The expressions of |
|
The mRNA expression levels of genes associated with mitophagy. The expressions of |
|
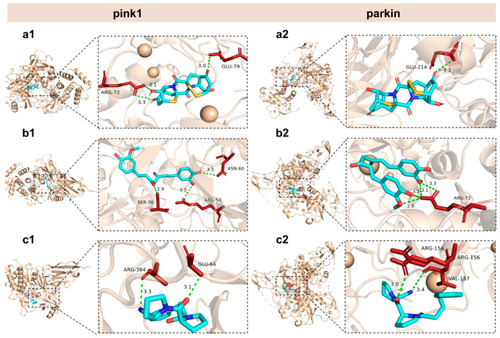
General and local perspectives of docking simulation of interactions between ligands and receptors, with pink1 and parkin being the receptors considered. Epicoccin A ( |
|
Transcriptome analysis. KEGG enrichment analysis of DEGs in MPTP vs. control ( |
|
mRNA expression levels of genes related to the mTOR/FoxO signaling pathway. The expressions of |
|
The proposed mechanism underlying the anti-PD effect of epicoccin A. Epicoccin A co-treatments can reverse the abnormal expressions of genes related to neuronal development, contributing to the improvement of neuronal damage in PD. Co-treatments with epicoccin A improved the aberrant gene expressions in the mTOR/FoxO signaling pathway, which might activate pink1/parkin-dependent mitophagy. This process facilitated the degradation of damaged mitochondria and α-synuclein fibrils, thereby inhibiting the formation of LBs. Moreover, epicoccin A co-treatments can inhibit oxidative stress by reducing ROS accumulation, further enhancing mitophagy and consequently alleviating the onset and development of PD. |
|
The experimental workflow chart. Larvae at 24 hpf were co-treated with MPTP and each of three different concentrations of epicoccin A from 24 hpf to either 96 hpf or 120 hpf. The developmental assessments of DA neurons, the nervous system, and the neural vasculature were conducted at 96 hpf. At 120 hpf, treated zebrafish were subjected to evaluations of locomotor behavior, ROS generation, expressions of genes related to PD, neurodevelopment, mitophagy, and oxidative stress, as well as transcriptome analysis. |