- Title
-
Genetic evidence for differential functions of figla and nobox in zebrafish ovarian differentiation and folliculogenesis
- Authors
- Wu, K., Zhai, Y., Qin, M., Zhao, C., Ai, N., He, J., Ge, W.
- Source
- Full text @ Commun Biol
|
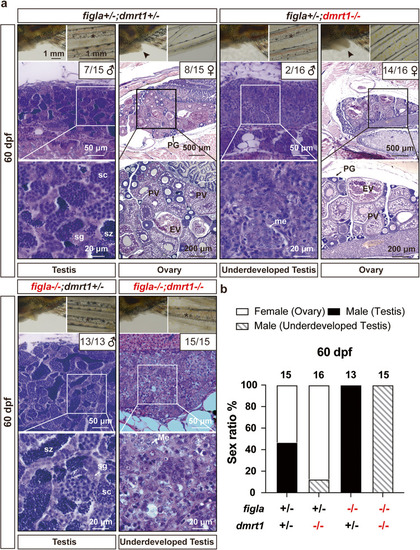
Phenotype analysis of different genotypes of |
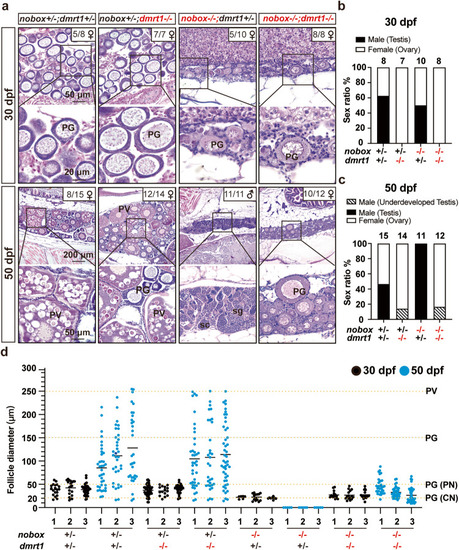
|
Rescue of the all-male phenotype of |
|
Long-term degeneration of the double mutant ovaries. |
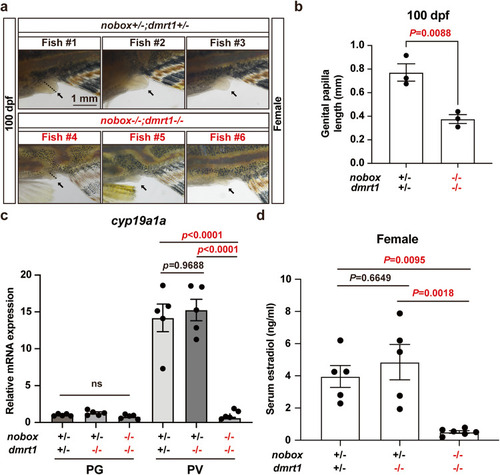
|
Evidence for estrogen deficiency in double mutant females. |
|
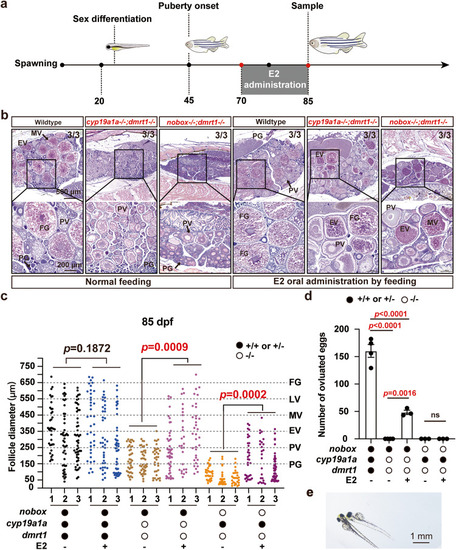
Ovarian growth and follicle development in the triple mutant of |
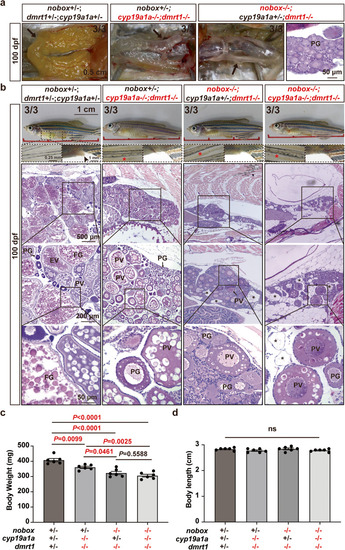
|
Rescue of vitellogenic growth in the double mutants ( |
|
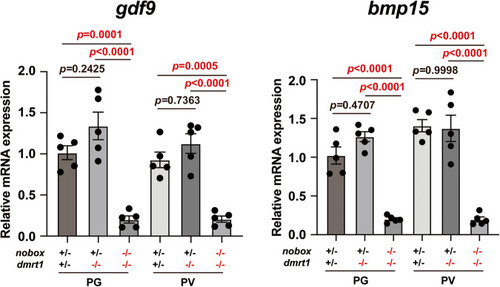
Expression of Total RNA was extracted from the isolated PG and PV follicles and reverse transcribed into cDNA for real-time PCR analysis. Each data point represents PG or PV follicles isolated and pooled from two fish for each genotype, totaling 10 fish ( |
|
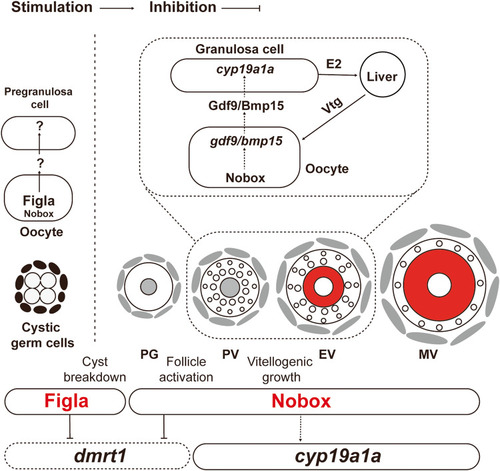
A working model on differential roles of Figla and Nobox in zebrafish folliculogenesis. Figla plays a critical role in controlling follicle formation in the event of cyst breakdown or follicle assembly, while Nobox is more involved in regulating follicle development after its formation, including such events as follicle activation (PG-PV transition) and vitellogenic growth (PV-EV transition). Nobox controls vitellogenic growth by regulating aromatase ( |