- Title
-
A novel role for the chloride intracellular channel protein Clic5 in ciliary function
- Authors
- Ott, E., Hoff, S., Indorf, L., Ditengou, F.A., Müller, J., Renschler, G., Lienkamp, S.S., Kramer-Zucker, A., Bergmann, C., Epting, D.
- Source
- Full text @ Sci. Rep.
|
Expression analyses of |
|
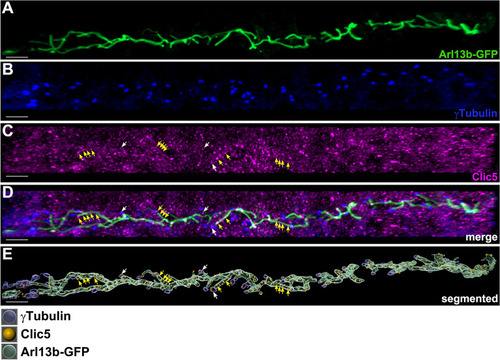
Subcellular localization analyses of Clic5 in the pronephric tubule of zebrafish. EXPRESSION / LABELING:
|
|
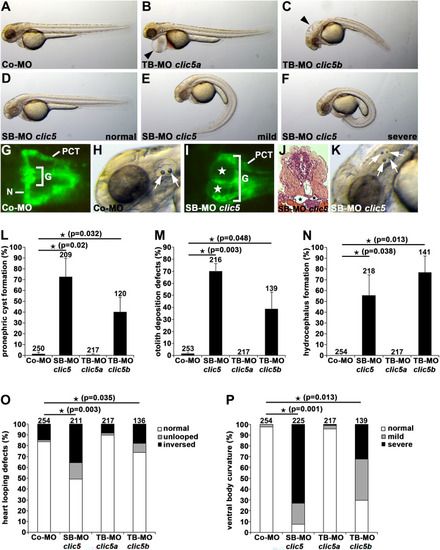
Clic5 knockdown analyses of cilia-related phenotypes in zebrafish. PHENOTYPE:
|
|
Analyses of Clic5 in ciliogenesis, and in ciliary and glomerular function in zebrafish. |
|
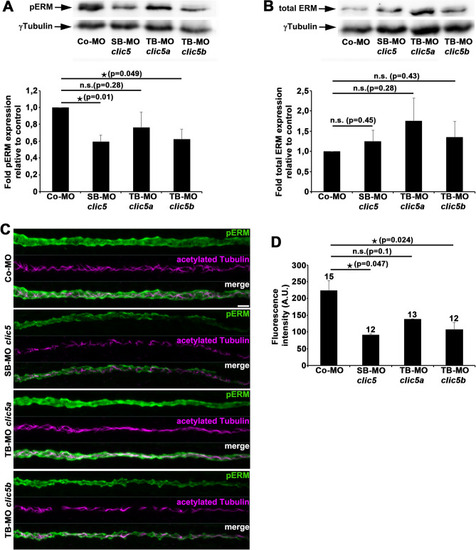
Analyses of pERM and total ERM levels after knockdown of Clic5, Clic5a or Clic5b. |