- Title
-
Apollo-NADP+ reveals in vivo adaptation of NADPH/NADP+ metabolism in electrically activated pancreatic β cells
- Authors
- Bui, C.V., Boswell, C.W., Ciruna, B., Rocheleau, J.V.
- Source
- Full text @ Sci Adv
|
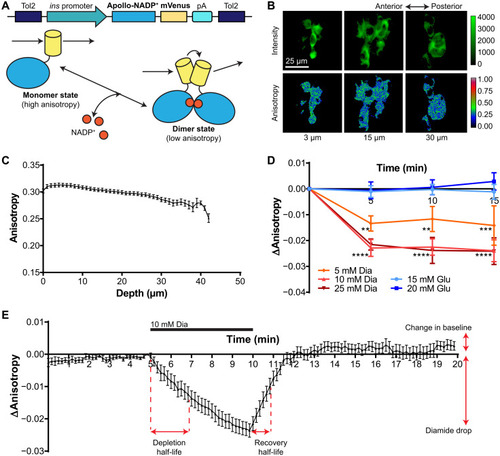
In vivo imaging of fluorescence anisotropy controls expressed in pancreatic β cells of 5-dpf zebrafish embryos. ( |
|
In vivo imaging of mVenus-tagged Apollo-NADP+ expressed in pancreatic β cells of 5-dpf zebrafish embryos. ( |
|
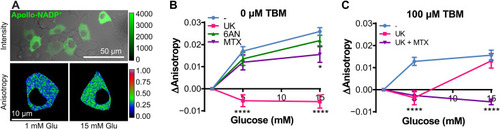
Selective inhibition of NADP+ reduction pathways in unstressed and stressed pancreatic β cells of 5-dpf zebrafish embryos. ( |
|
Selective inhibition of NADP+ reduction pathways in unstressed and stressed INS1E pancreatic β cells. ( |
|
Investigating potential mechanisms of folate cycle activation during chronic stress in INS1E β cells. ( |