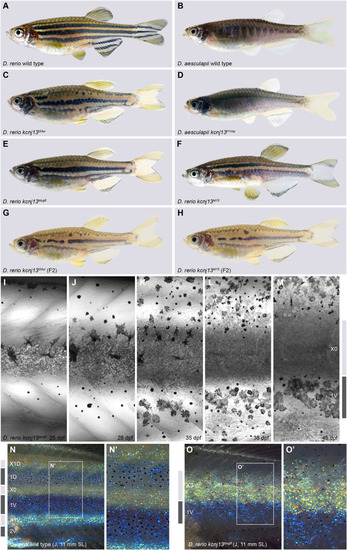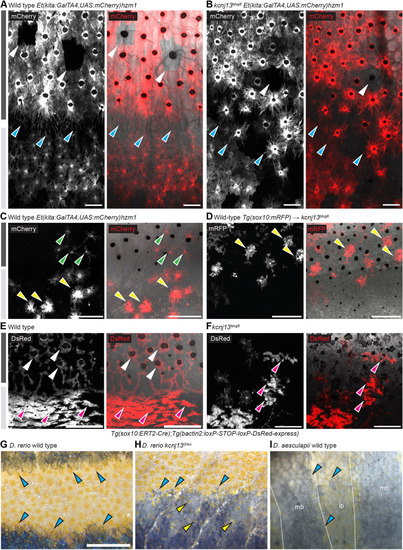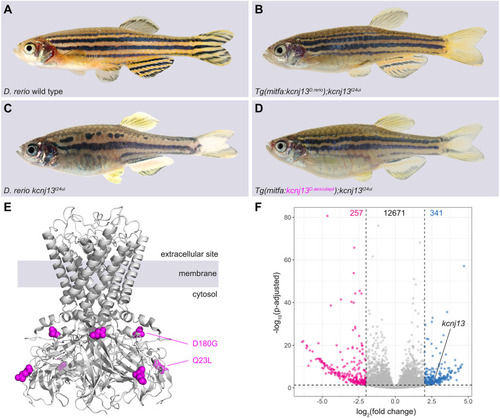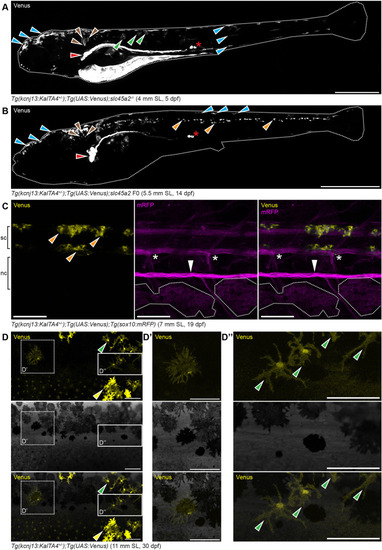
Endogenous kcnj13 expression during D. rerio development. (A) Heterozygous KalTA4::Venus reporter larva (n>50) showing signals in melanophores in the head and tail regions (cyan arrowheads), xanthophores (green arrowheads), hindbrain (brown arrowheads), along the entire pronephros (red arrowhead), including corpuscles of Stannius (red asterisk), and the yolk. 4 mm standard length (SL), 5 dpf, sagittal view, images of four positions along the A-P axis combined into one composite. (B) Similar expression patterns can be observed in larva 1 week older (n>25), with additional signals in the spinal cord (orange arrowheads). These signals persist throughout further development. 5.5 mm SL, 14 dpf, sagittal view, images of five combined into one composite. (C) Venus expression does not overlap with locations of the pigment cell stem cells at the DRGs (marked by white asterisks) in reporter larva (n>10). Iridophore patches in the skin indicated with white dotted outlines, lateral line nerve marked with a white arrowhead; presumptive neurons in the spinal cord marked with orange arrowheads. nc, notochord; sc, spinal cord. 7 mm SL, 19 dpf. (D-D″) During and after the consolidation of the stripes in wild types (n>10, see Fig. 1I-O), Venus expression can be detected in only a minority of melanophores (D′) and xanthophores (D″) in the skin at any given time point. Green arrowheads indicate stellate and Venus-positive xanthophores in the dark stripe, yellow arrowheads indicate compact, pigmented and Venus-positive xanthophores in the light stripe. 11 mm SL, 30 dpf. Scale bars: 500 µm (A); 1 mm (B); 100 µm (C-D″).
|