- Title
-
Human Mutated MYOT and CRYAB Genes Cause a Myopathic Phenotype in Zebrafish
- Authors
- Cannone, E., Guglielmi, V., Marchetto, G., Tobia, C., Gnutti, B., Cisterna, B., Tonin, P., Barbon, A., Vattemi, G., Schiavone, M.
- Source
- Full text @ Int. J. Mol. Sci.
|
Light microscopy of the muscle biopsy from control subject ( |
|
Immunofluorescence staining of the muscle biopsy from control subject and patient with p.Gly154Ser |
|
Transmission electron micrographs of the muscle biopsy from control subject ( |
|
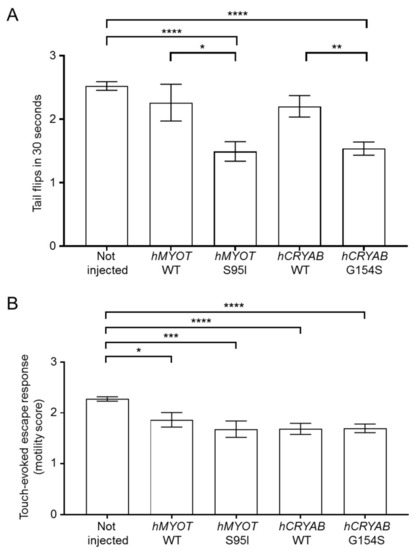
Effects on zebrafish motor behavior of both wildtype and mutant |
|
Effects on muscle fiber structure and development of both wildtype and mutant |
|
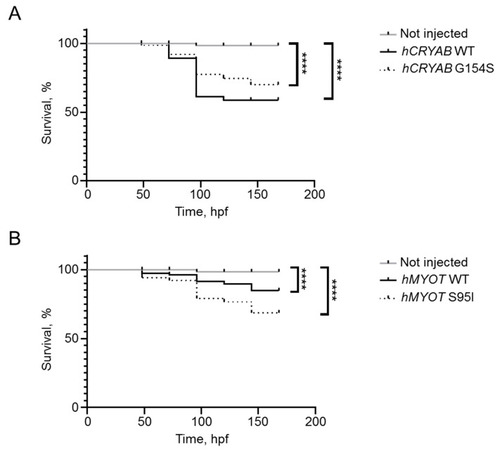
Effects on fish survival of both wildtype and mutant |
|
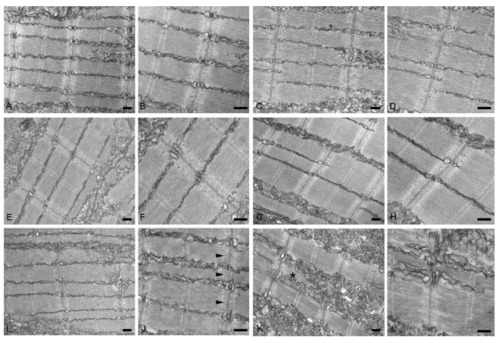
Transmission electron micrographs of no-injected ( |
|
Whole-mount immunofluorescence shows highly fluorescent dots in zebrafish embryos injected with both wt and mutated |
|
Lightsheet microscopy shows highly fluorescent dots in zebrafish embryos injected with both wt and mutated |