- Title
-
Direct BMP signaling to chordoblasts is required for the initiation of segmented notochord sheath mineralization in zebrafish vertebral column development
- Authors
- Pogoda, H.M., Riedl-Quinkertz, I., Hammerschmidt, M.
- Source
- Full text @ Front Endocrinol (Lausanne)
|
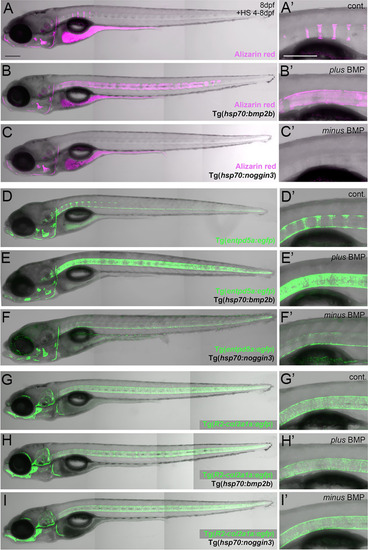
BMP regulates mineralization and entpd5a expression along the notochord. (A–I) Lateral views of zebrafish larvae shown in merged transmitted and fluorescent light channels with tissue staining and genotypes as indicated at an age of 8dpf. Alizarin red labels mineralized extracellular matrix (ECM) in magenta (A–C). entpd5a-expressing cells are visualized by Tg(entpd5a:egfp) in green (D–F), col2a1-expressing chondroblasts and chordoblasts by Tg(R2-col2a1a:egfp) in green (G–I). Magnified views of the region right dorsal to the swim bladder from corresponding larvae are shown in A’ o I’. All larvae have been heat-shocked once a day for 45 minutes at 40°C from 4-8 dpf. Note the ventral line of chordoblasts along the entire length of the notochord that remained EGFP positive after noggin3 overexpression (F, F’), pointing to BMP-independent regulation in this particular notochordal subdomain. Scale bars: 200 µm. |
|
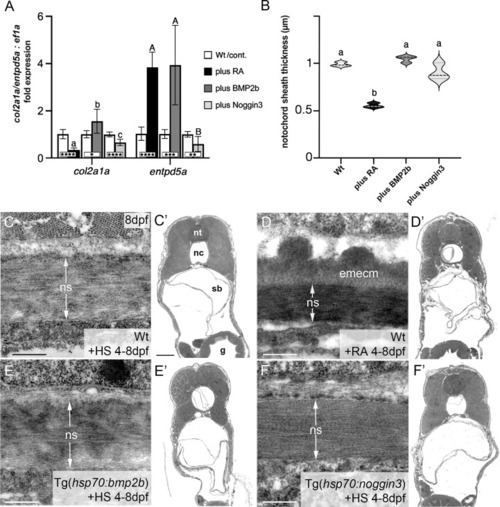
BMP has no major impact on col2a1 expression and notochord sheath formation. (A) qPCR results for the matrix-generating gene col2a1a and the matrix-mineralizing gene entpd5a after RA exposure, BMP2b or Noggin3 overexpression, respectively. See main text for exact experimental conditions. Each shaded column indicates the ratio of expression levels between an experimental group and its specific control group set to a value of 1 (white columns), with the respective statistical significances determined via Student’s t-test with Welch’s correction and indicated as * (significant with p<0.05), ** (significant with p<0.01), *** (significant with p<0.001) and **** (significant with p<0.0001). In addition, the letter subscript system (small letters for col2a1a; capital letters for entpd5a) is used to indicate significances of differences between the different experimental groups, with groups with the same letters being not significantly different according to Brown-Forsythe and Welch ANOVA tests (p < 0.0001) with post-hoc Dunnett’s T3 multiple comparison test (p < 0.05). (B) Quantitative analysis of notochord sheath thickness, based on measurements performed on TEM specimens as in (C–F). Listed conditions have been obtained via drug treatment (RA) or by the aforementioned heat-shock inducible transgenes; violin plots of N=3 biological samples per condition from independent spawnings/treatments, with each N representing the mean value from 5 different sections per specimen; broken and dotted lines indicate mean and quartiles, respectively; experimental groups labeled with identical superscript letters are not significantly different according to Student’s t-test with Welch’s correction, p < 0.05. (C–F) TEM micrographs of cross-sections through the abdominal notochord sheath from larvae with genotypes and treatments as indicated; differences in the darkness of the notochord sheath are due to differences in its mineralization levels in the different conditions. (C’–F’) show overviews of the corresponding semithin-sections indicating that the TEM images were taken at corresponding positions along the antero-posterior axis of the specimens. Scale bars: 0.5 µm (C–F), 50 µm (C’); g, gut; nc, notochord; ns, notochordal sheath; nt, neural tube; emecm, external mineralized extracellular matrix; sb, swim bladder. |
|
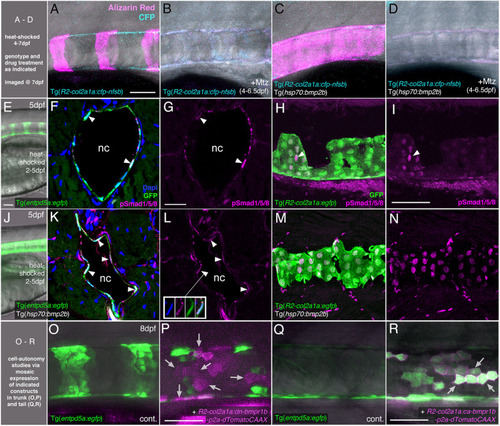
BMP directly targets chordoblasts to regulate Entp5a-mediated mineralization of the notochord sheath. (A–D) BMP induced hyper-mineralization of the notochord sheath requires an intact chordoblast layer. (E–N) Chordoblasts receive BMP signals as indicated by the presence of the responsible intracellular mediator pSmad1/5/8. Apart from chordoblasts, global overexpression of bmp2b via induction of Tg(hsp70:bmp2b) also resulted in the gain of pSmad1/5/8-positive cells within the myosepta, muscle tissue and the neural tube (K-N). (O–R) Mosaic chordoblast-specific blockage or activation of the intracellular BMP signaling cascade alters entpd5a expression autonomously in targeted notochord epithelium cells. (A–R) Maximum intensity projections of confocal images with merged channels showing notochords and their immediate surroundings in live whole-mounts (A–E, J, O–R) and on stained cryosections (F, H, K, M). (G, I, L, N) show corresponding single 555nm channels in magenta. (E, J) delineate overviews of those areas of heat-shocked larvae from which the sections presented in the corresponding rows were prepared. (A–P) have been assayed in the abdominal region at the level of the swim bladder, (Q) and (R) in the tail. (A–E, H–J, M–R) are lateral views with anterior to the left, (F, G, K, L) show transverse sections with dorsal upwards. Arrowheads in (F, G, I, K, L) point to exemplary chordoblasts with clear nuclear pSmad1/5/8 staining. Inset in (L) depicts magnified single channel images of pSmad1/5/8-positive chordoblast indicated in (L) by connected arrowhead. Arrows in (P) mark magenta-colored dn-bmp1b-p2a-dTomatoCAAX expressing cells that are located in a usually entpd5a-positive chordoblast cluster (compare with (O)), but that do not express the entpd5a:egfp transgene when BMP signal reception is blocked (N=5; n=28/39 dTomatoCAAX-positive, but EGFP-negative cells). Arrows in (R) point at a prominent group of chordoblasts that are triple positive for EGFP, the constitutively active BMP receptor Bmpr1b and dTomatoCAAX (N=2, 48 dTomatoCaax and EGFP double-positive of 53 dTomatoCAAX expressing chordoblasts). Ages, treatments, genotypes and color-coded markers as indicated. Scale bars: 50 µm (A, E, I, O, P); 25 µm (G). Mtz, Metronidazole; nc, notochord. |
|
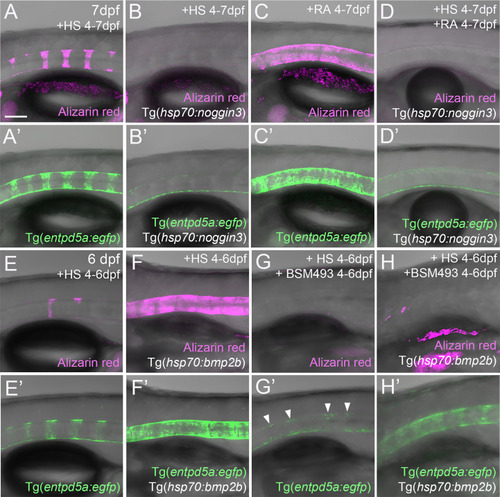
RA cannot promote notochord sheath mineralization when BMP signaling is blocked during initiation phases of mineralization. (A–D’) RA fails to induce hyper-mineralization of the notochord sheath while BMP signaling is blocked. (E–H’) BMP induced ectopic entpd5a activation cannot be hindered by interfering with the intra-cellular signal transduction of the RA pathway. (A–H’) Lateral views of transgenic zebrafish larvae shown in merged transmitted and fluorescent light channels with tissue stainings, genotypes and treatments as indicated at an age of 7dpf (A–D’) or 6dpf (E–H’). Heat-shocks were performed once a day for 45 minutes at 40°C. Arrowheads in (G’) point to faintly EGFP-positive chordoblasts in areas of presumably segmented Tg(entpd5a:egfp) expression. Image-pairs X/X’ show identical specimens. Scale bar (shown in A, applying to all panels): 100 µm. |
|
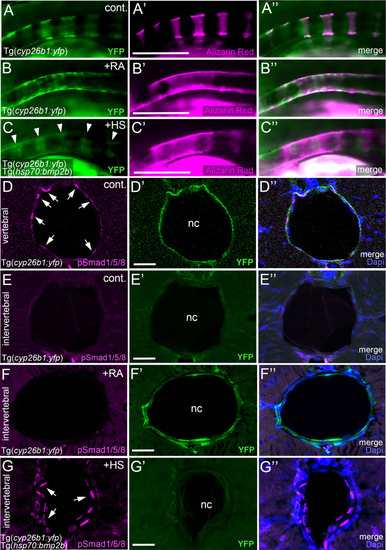
BMP and RA signaling do not act via each other. (A–C’’) BMP-induced hyper-mineralization is not accompanied by an ectopic activation of the RA signaling reporter Tg(cyp26b1:yfp) in the notochord epithelium. Lateral views of notochords immediately dorsal to the swim bladder, anterior to the left. Images were taken by conventional fluorescent light microscopy and are shown as separate 488nm (A–C), 555nm (A’–C’) channels and with both channels merged (A’’–C’’). The cyp26b1:yfp transgene (A–C, A’’–C’’, green) labels chordoblasts and serves as a readout for RA-responsiveness. Alizarin red stains the mineralized notochord sheath (A’–C’, A’’–C’’ magenta). Note the remaining segmented YFP signal after global overexpression of BMP2b (C, arrowheads). (D–G’’) Exposure of larvae to 1 µM RA does not cause ectopic pSmad1/5/8 appearance at intervertebral positions of the chordoblast layer. Cross-sections through the abdominal notochord of 7 dpf control Tg(cyp26b1:yfp) (D–E’’), RA-treated Tg(cyp26b1:yfp) (F–F’’), and heat-shocked Tg(cyp26b1:yfp), Tg(hsp70:bmp2b) (G–G’’) larvae co-stained via immunofluorescence labeling for the activated/phosphorylated BMP signal transducer pSmad1/5/8 and for YFP, encoded by the RA responder transgene. In untreated controls, pSmad1/5/8 reactivity is restricted to vertebral segments co-expressing the RA reporter transgene (D–D’’) but absent from intervertebral spaces lacking Tg(cyp26b1:yfp) expression (E–E’’). Upon RA-treatment, such intervertebral domains (identified as such by the lack of pSmad1/5/8 activity) display ectopic Tg(cyp26b1:yfp) activity, although RA fails to induce ectopic pSmad1/5/8 reactivity (F-F’’). In contrast, as a positive control, ectopic pSmad1/5/8 reactivity in intervertebral regions, identified as such by the lack of Tg(cyp26b1:yfp) activity, is obtained by heat shock-induced ubiquitous Tg(hsp70:bmp2b) expression (G-G’’). Images represent single confocal slices shown as separate channels 555nm in magenta (D–G), 488nm in green (D’–G’) and merged with Dapi (D’’–G’’). Arrows in (D, G) point at exemplary pSmad1/5/8 positive chordoblasts. Whole figure: Genotype, age, labeling and treatments as indicated. Heat-shocks were performed once a day for 45 minutes at 40°C from 4-7 dpf. RA was applied constantly from 4-7 dpf. Scale bars: 200 µm (B); 20 µm (D’–G’). cont., vehicle-treated control; nc, notochord; HS, heat shock; RA, Retinoic acid. |
|
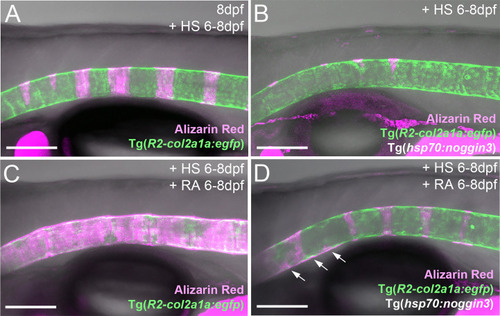
RA can promote notochord sheath mineralization independently of BMP signaling at later stages of vertebral column formation. (A–D) Blockage of BMP signaling with synchronous activation of the RA pathway from six to eight dpf results in larvae with less severe disrupted notochord sheath mineralization while chordoblasts show clear marks of RA responsiveness (decreased Tg(R2-col2a1a:egfp) activity and less distinct cellular morphology). Lateral views of transgenic zebrafish larvae shown in merged transmitted and fluorescent light channels with tissue staining, genotypes and treatments as indicated at an age of 8dpf (A–D). Heat-shocks were performed once a day for 45 minutes at 40°C. Arrows in (D) point at zones of ectopic mineralization. Scale bars: 150 µm. |
|
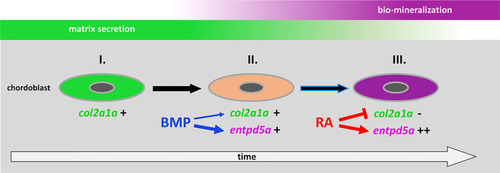
Proposed model for the consecutive roles of BMP and RA to induce the stepwise progression of chordoblasts from solely matrix-producing towards solely matrix-mineralizing cells. I. Chordoblast contribution to chordacentra formation starts with collagen II-based matrix (notochord sheath) production. II. Next, cells progress into a matrix-secreting and -mineralizing transitory stage induced by BMP, with an induction of entpd5a expression, required for matrix mineralization (thick blue arrow) and with some minor positive effect on col2a1a expression (thin blue arrow). III. Lastly, RA signaling enhances and maintains entpd5a transcription (thick red arrow), thereby reaching Entpd5a levels that allow for sustained bio-mineralization of the notochord sheath. At the same time RA represses col2a1a transcription to attenuate matrix secretion. Chordoblasts can only enter step III after they have experienced BMP signaling beforehand (black arrow with blue lining). |